The safety, pharmacokinetics, and effects of LGD-4033, a novel nonsteroidal oral, selective androgen receptor modulator, in healthy young men
- PMID: 22459616
- PMCID: PMC4111291
- DOI: 10.1093/gerona/gls078
The safety, pharmacokinetics, and effects of LGD-4033, a novel nonsteroidal oral, selective androgen receptor modulator, in healthy young men
Abstract
Background: Concerns about potential adverse effects of testosterone on prostate have motivated the development of selective androgen receptor modulators that display tissue-selective activation of androgenic signaling. LGD-4033, a novel nonsteroidal, oral selective androgen receptor modulator, binds androgen receptor with high affinity and selectivity. Objectives. To evaluate the safety, tolerability, pharmacokinetics, and effects of ascending doses of LGD-4033 administered daily for 21 days on lean body mass, muscle strength, stair-climbing power, and sex hormones.
Methods: In this placebo-controlled study, 76 healthy men (21-50 years) were randomized to placebo or 0.1, 0.3, or 1.0 mg LGD-4033 daily for 21 days. Blood counts, chemistries, lipids, prostate-specific antigen, electrocardiogram, hormones, lean and fat mass, and muscle strength were measured during and for 5 weeks after intervention.
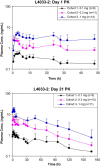
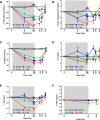
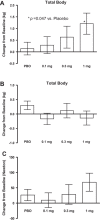
Results: LGD-4033 was well tolerated. There were no drug-related serious adverse events. Frequency of adverse events was similar between active and placebo groups. Hemoglobin, prostate-specific antigen, aspartate aminotransferase, alanine aminotransferase, or QT intervals did not change significantly at any dose. LGD-4033 had a long elimination half-life and dose-proportional accumulation upon multiple dosing. LGD-4033 administration was associated with dose-dependent suppression of total testosterone, sex hormone-binding globulin, high density lipoprotein cholesterol, and triglyceride levels. follicle-stimulating hormone and free testosterone showed significant suppression at 1.0-mg dose only. Lean body mass increased dose dependently, but fat mass did not change significantly. Hormone levels and lipids returned to baseline after treatment discontinuation.
Conclusions: LGD-4033 was safe, had favorable pharmacokinetic profile, and increased lean body mass even during this short period without change in prostate-specific antigen. Longer randomized trials should evaluate its efficacy in improving physical function and health outcomes in select populations.
Figures
References
-
- Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107:123–136. - PubMed
-
- Roy TA, Blackman MR, Harman SM, Tobin JD, Schrager M, Metter EJ. Interrelationships of serum testosterone and free testosterone index with FFM and strength in aging men. Am J Physiol Endocrinol Metab. 2002;283:E284–E294. - PubMed
-
- Melton LJ, III, Khosla S, Crowson CS, O’Connor MK, O’Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48:625–630. - PubMed
-
- Bassey EJ, Fiatarone MA, O’Neill EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci (Lond) 1992;82:321–327. - PubMed
-
- Lexell J, Downham D, Sjostrom M. Distribution of different fibre types in human skeletal muscles. A statistical and computational study of the fibre type arrangement in m. vastus lateralis of young, healthy males. J Neurol Sci. 1984;65:353–365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

