Modifications in erythrocyte membrane zeta potential by Plasmodium falciparum infection
- PMID: 22459624
- PMCID: PMC3361589
- DOI: 10.1016/j.exppara.2012.03.005
Modifications in erythrocyte membrane zeta potential by Plasmodium falciparum infection
Abstract
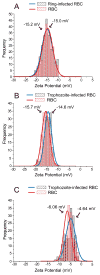
The zeta potential (ZP) is an electrochemical property of cell surfaces that is determined by the net electrical charge of molecules exposed at the surface of cell membranes. Membrane proteins contribute to the total net electrical charge of cell surfaces and can alter ZP through variation in their copy number and changes in their intermolecular interactions. Plasmodium falciparum extensively remodels its host red blood cell (RBC) membrane by placing 'knob'-like structures at the cell surface. Using an electrophoretic mobility assay, we found that the mean ZP of human RBCs was -15.7 mV. In RBCs infected with P. falciparum trophozoites ('iRBCs'), the mean ZP was significantly lower (-14.6 mV, p<0.001). Removal of sialic acid from the cell surface by neuraminidase treatment significantly decreased the ZP of both RBCs (-6.06 mV) and iRBCs (-4.64 mV). Parasite-induced changes in ZP varied by P. falciparum clone and the presence of knobs on the iRBC surface. Variations in ZP values were accompanied by altered binding of iRBCs to human microvascular endothelial cells (MVECs). These data suggest that parasite-derived knob proteins contribute to the ZP of iRBCs, and that electrostatic and hydrophobic interactions between iRBC and MVEC membranes are involved in cytoadherence.
Published by Elsevier Inc.
Figures
References
-
- Aikawa M, Kamanura K, Shiraishi S, Matsumoto Y, Arwati H, Torii M, Ito Y, Takeuchi T, Tandler B. Membrane knobs of unfixed Plasmodium falciparum infected erythrocytes: new findings as revealed by atomic force microscopy and surface potential spectroscopy. Exp Parasitol. 1996;84:339–343. - PubMed
-
- Baruch DI, Ma XC, Singh HB, Bi X, Pasloske BL, Howard RJ. Identification of a region of PfEMP1 that mediates adherence of Plasmodium falciparum infected erythrocytes to CD36: conserved function with variant sequence. Blood. 1997;90:3766–3775. - PubMed
-
- Bostrom M, Williams DR, Ninham BW. Specific ion effects: why DLVO theory fails for biology and colloid systems. Phys Rev Lett. 2001;87:168103. - PubMed
-
- Buffet PA, Gamain B, Scheidig C, Baruch D, Smith JD, Hernandez-Rivas R, Pouvelle B, Oishi S, Fujii N, Fusai T, Parzy D, Miller LH, Gysin J, Scherf A. Plasmodium falciparum domain mediating adhesion to chondroitin sulfate A: a receptor for human placental infection. Proc Natl Acad Sci U S A. 1999;96:12743–12748. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

