Apoptosis-induced mitochondrial dysfunction causes cytoplasmic lipid droplet formation
- PMID: 22460322
- PMCID: PMC3422477
- DOI: 10.1038/cdd.2012.34
Apoptosis-induced mitochondrial dysfunction causes cytoplasmic lipid droplet formation
Abstract
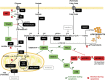
A characteristic of apoptosis is the rapid accumulation of cytoplasmic lipid droplets, which are composed largely of neutral lipids. The proton signals from these lipids have been used for the non-invasive detection of cell death using magnetic resonance spectroscopy. We show here that despite an apoptosis-induced decrease in the levels and activities of enzymes involved in lipogenesis, which occurs downstream of p53 activation and inhibition of the mTOR signaling pathway, the increase in lipid accumulation is due to increased de novo lipid synthesis. This results from inhibition of mitochondrial fatty acid β-oxidation, which coupled with an increase in acyl-CoA synthetase activity, diverts fatty acids away from oxidation and into lipid synthesis. The inhibition of fatty acid oxidation can be explained by a rapid rise in mitochondrial membrane potential and an attendant increase in the levels of reactive oxygen species.
Figures
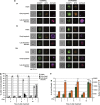


 ) and p21 (
) and p21 ( ) obtained by quantitative polymerase chain reaction (PCR). Results are expressed in relation to their initial levels (untreated controls). Actin expression levels were used to normalize the results (mean±S.E.M.; *P<0.01, **P<0.001, n=3). (c) Expression levels of ACLY (
) obtained by quantitative polymerase chain reaction (PCR). Results are expressed in relation to their initial levels (untreated controls). Actin expression levels were used to normalize the results (mean±S.E.M.; *P<0.01, **P<0.001, n=3). (c) Expression levels of ACLY ( ), ACC1 (
), ACC1 ( ) and FASN (
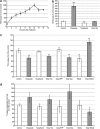
) and FASN ( ) obtained by quantitative PCR. Results were normalized to the actin levels in each sample and expressed relative to the untreated control (mean±S.E.M.; *P<0.01, **P<0.001, n=3). (d) Western blot analysis of the levels of ACLY, p-ACLY (Ser454), ACC1, p-ACC1 (Ser79), FASN and ACS. Actin was used as an internal protein loading control. A representative sample is shown for each protein
) obtained by quantitative PCR. Results were normalized to the actin levels in each sample and expressed relative to the untreated control (mean±S.E.M.; *P<0.01, **P<0.001, n=3). (d) Western blot analysis of the levels of ACLY, p-ACLY (Ser454), ACC1, p-ACC1 (Ser79), FASN and ACS. Actin was used as an internal protein loading control. A representative sample is shown for each protein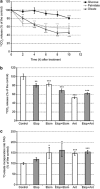
References
-
- Buchakjian MR, Kornbluth S. The engine driving the ship: metabolic steering of cell proliferation and death. Nat Rev Mol Cell Biol. 2010;11:715–727. - PubMed
-
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. - PubMed
-
- Bensaad K, Tsuruta A, Selak MA, Vidal MNC, Nakano K, Bartrons R, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. - PubMed
-
- Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

