New insights into the dynamic regulation of water and acid-base balance by renal epithelial cells
- PMID: 22460710
- PMCID: PMC3362000
- DOI: 10.1152/ajpcell.00085.2012
New insights into the dynamic regulation of water and acid-base balance by renal epithelial cells
Abstract
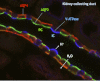
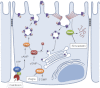
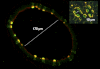
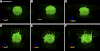
Maintaining tight control over body fluid and acid-base homeostasis is essential for human health and is a major function of the kidney. The collecting duct is a mosaic of two cell populations that are highly specialized to perform these two distinct processes. The antidiuretic hormone vasopressin (VP) and its receptor, the V2R, play a central role in regulating the urinary concentrating mechanism by stimulating accumulation of the aquaporin 2 (AQP2) water channel in the apical membrane of collecting duct principal cells. This increases epithelial water permeability and allows osmotic water reabsorption to occur. An understanding of the basic cell biology/physiology of AQP2 regulation and trafficking has informed the development of new potential treatments for diseases such as nephrogenic diabetes insipidus, in which the VP/V2R/AQP2 signaling axis is defective. Tubule acidification due to the activation of intercalated cells is also critical to organ function, and defects lead to several pathological conditions in humans. Therefore, it is important to understand how these "professional" proton-secreting cells respond to environmental and cellular cues. Using epididymal proton-secreting cells as a model system, we identified the soluble adenylate cyclase (sAC) as a sensor that detects luminal bicarbonate and activates the vacuolar proton-pumping ATPase (V-ATPase) via cAMP to regulate tubular pH. Renal intercalated cells also express sAC and respond to cAMP by increasing proton secretion, supporting the hypothesis that sAC could function as a luminal sensor in renal tubules to regulate acid-base balance. This review summarizes recent advances in our understanding of these fundamental processes.
Figures

References
-
- Al-Awqati Q. 2007 Homer W. Smith award: control of terminal differentiation in epithelia. J Am Soc Nephrol 19: 443–449, 2008 - PubMed
-
- Al-Awqati Q. Terminal differentiation in epithelia: the role of integrins in hensin polymerization. Annu Rev Physiol 73: 401–412, 2011 - PubMed
-
- Al-Awqati Q, Gluck S, Reeves W, Cannon C. Regulation of proton transport in urinary epithelia. J Exp Biol 106: 135–141, 1983 - PubMed
-
- Alzamora R, Thali RF, Gong F, Smolak C, Li H, Baty CJ, Bertrand CA, Auchli Y, Brunisholz RA, Neumann D, Hallows KR, Pastor-Soler NM. PKA regulates vacuolar H+-ATPase localization and activity via direct phosphorylation of the a subunit in kidney cells. J Biol Chem 285: 24676–24685, 2010 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K01 DK073266/DK/NIDDK NIH HHS/United States
- DK-075940/DK/NIDDK NIH HHS/United States
- DK-38452/DK/NIDDK NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- DK-HD40793/DK/NIDDK NIH HHS/United States
- DK-42956/DK/NIDDK NIH HHS/United States
- K08 DK075940/DK/NIDDK NIH HHS/United States
- R01 HD040793/HD/NICHD NIH HHS/United States
- P01 DK038452/DK/NIDDK NIH HHS/United States
- R37 DK042956/DK/NIDDK NIH HHS/United States
- R01 DK042956/DK/NIDDK NIH HHS/United States
- DK-73266/DK/NIDDK NIH HHS/United States
- R01 DK097124/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

