Knockout of Mkp-1 exacerbates colitis in Il-10-deficient mice
- PMID: 22461024
- PMCID: PMC3378166
- DOI: 10.1152/ajpgi.00018.2012
Knockout of Mkp-1 exacerbates colitis in Il-10-deficient mice
Abstract
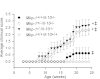
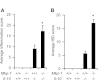
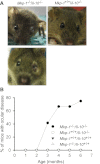
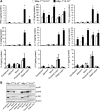
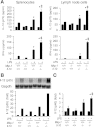
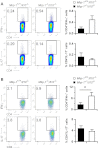
Il-10-deficient mice develop colitis associated with exaggerated Th1/Th17 responses and are a valuable model of inflammatory bowel disease. Mkp-1 is a major negative regulator of MAPKs, and its expression is enhanced by IL-10. To understand the role of Mkp-1 in the regulation of intestinal mucosal immune responses, we studied the effect of Mkp-1 deletion on the pathogenesis of colitis in Il-10(-/-) mice. We found that knockout of Mkp-1 on an Il-10(-/-) background accelerated the development of colitis. Compared with Il-10(-/-) mice, colitis not only appeared earlier but also was more severe in Il-10(-/-)/Mkp-1(-/-) mice. Il-10(-/-) mice exhibited a mild intestinal inflammation in the specific pathogen-free environment, and rectal prolapse rarely appeared before 6 mo of age. In contrast, the majority of Il-10(-/-)/Mkp-1(-/-) mice developed severe colitis rapidly and presented with rectal prolapse after only 2-3 mo. The colon of Il-10(-/-)/Mkp-1(-/-) mice showed diffuse transmural chronic inflammation and mucosal hyperplasia, with significantly more proliferating crypt epithelial cells than those of Il-10(-/-) mice. In addition to the severe colitis, Il-10(-/-)/Mkp-1(-/-) mice also developed conjunctivitis and blepharitis. The colon of Il-10(-/-)/Mkp-1(-/-) mice contained significantly higher levels of proinflammatory cytokines and exhibited greater MAPK activities than did the colon of Il-10(-/-) mice. Splenocytes and lymphocytes from Il-10(-/-)/Mkp-1(-/-) mice produced higher levels of Th1 cytokines ex vivo upon activation than did cells from Il-10(-/-) mice. Our studies support a pivotal role of Mkp-1 as a negative regulator of mucosal immune responses and highlight its protective function against inflammatory bowel disease.
Figures




Similar articles
-
Mast cell deficiency exacerbates inflammatory bowel symptoms in interleukin-10-deficient mice.World J Gastroenterol. 2014 Jul 21;20(27):9106-15. doi: 10.3748/wjg.v20.i27.9106. World J Gastroenterol. 2014. PMID: 25083083 Free PMC article.
-
Memory/effector (CD45RB(lo)) CD4 T cells are controlled directly by IL-10 and cause IL-22-dependent intestinal pathology.J Exp Med. 2011 May 9;208(5):1027-40. doi: 10.1084/jem.20102149. Epub 2011 Apr 25. J Exp Med. 2011. PMID: 21518800 Free PMC article.
-
Gut Epithelial Vitamin D Receptor Regulates Microbiota-Dependent Mucosal Inflammation by Suppressing Intestinal Epithelial Cell Apoptosis.Endocrinology. 2018 Feb 1;159(2):967-979. doi: 10.1210/en.2017-00748. Endocrinology. 2018. PMID: 29228157 Free PMC article.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Insights from mouse models of colitis.J Leukoc Biol. 2000 Mar;67(3):267-78. doi: 10.1002/jlb.67.3.267. J Leukoc Biol. 2000. PMID: 10733087 Review.
Cited by
-
Role of Dual-Specificity Phosphatase 1 in Glucocorticoid-Driven Anti-inflammatory Responses.Front Immunol. 2019 Jun 26;10:1446. doi: 10.3389/fimmu.2019.01446. eCollection 2019. Front Immunol. 2019. PMID: 31316508 Free PMC article. Review.
-
The E3 Ubiquitin Protein Ligase LINCR Amplifies the TLR-Mediated Signals through Direct Degradation of MKP1.Cells. 2024 Apr 15;13(8):687. doi: 10.3390/cells13080687. Cells. 2024. PMID: 38667302 Free PMC article.
-
Methylprednisolone Promotes Mycobacterium smegmatis Survival in Macrophages through NF-κB/DUSP1 Pathway.Microorganisms. 2023 Mar 16;11(3):768. doi: 10.3390/microorganisms11030768. Microorganisms. 2023. PMID: 36985341 Free PMC article.
-
Epithelial Toll-like receptors and their role in gut homeostasis and disease.Nat Rev Gastroenterol Hepatol. 2020 May;17(5):263-278. doi: 10.1038/s41575-019-0261-4. Epub 2020 Feb 26. Nat Rev Gastroenterol Hepatol. 2020. PMID: 32103203 Review.
-
Sequestering HMGB1 via DNA-conjugated beads ameliorates murine colitis.PLoS One. 2014 Aug 15;9(8):e103992. doi: 10.1371/journal.pone.0103992. eCollection 2014. PLoS One. 2014. PMID: 25127031 Free PMC article.
References
-
- Anderson CA, Boucher G, Lees CW, Franke A, D'Amato M, Taylor KD, Lee JC, Goyette P, Imielinski M, Latiano A, Lagace C, Scott R, Amininejad L, Bumpstead S, Baidoo L, Baldassano RN, Barclay M, Bayless TM, Brand S, Buning C, Colombel JF, Denson LA, de Vos M, Dubinsky M, Edwards C, Ellinghaus D, Fehrmann RS, Floyd JA, Florin T, Franchimont D, Franke L, Georges M, Glas J, Glazer NL, Guthery SL, Haritunians T, Hayward NK, Hugot JP, Jobin G, Laukens D, Lawrance I, Lemann M, Levine A, Libioulle C, Louis E, McGovern DP, Milla M, Montgomery GW, Morley KI, Mowat C, Ng A, Newman W, Ophoff RA, Papi L, Palmieri O, Peyrin-Biroulet L, Panes J, Phillips A, Prescott NJ, Proctor DD, Roberts R, Russell R, Rutgeerts P, Sanderson J, Sans M, Schumm P, Seibold F, Sharma Y, Simms LA, Seielstad M, Steinhart AH, Targan SR, van den Berg LH, Vatn M, Verspaget H, Walters T, Wijmenga C, Wilson DC, Westra HJ, Xavier RJ, Zhao ZZ, Ponsioen CY, Andersen V, Torkvist L, Gazouli M, Anagnou NP, Karlsen TH, Kupcinskas L, Sventoraityte J, Mansfield JC, Kugathasan S, Silverberg MS, Halfvarson J, Rotter JI, Mathew CG, Griffiths AM, Gearry R, Ahmad T, Brant SR, Chamaillard M, Satsangi J, Cho JH, Schreiber S, Daly MJ, Barrett JC, Parkes M, Annese V, Hakonarson H, Radford-Smith G, Duerr RH, Vermeire S, Weersma RK, Rioux JD. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet 43: 246– 252, 2011 - PMC - PubMed
-
- Ardizzone S, Puttini PS, Cassinotti A, Porro GB. Extraintestinal manifestations of inflammatory bowel disease. Dig Liver Dis 40, Suppl 2: S253– S259, 2008 - PubMed
-
- Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy—review of a new approach. Pharmacol Rev 55: 241– 269, 2003 - PubMed
-
- Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM, Bitton A, Dassopoulos T, Datta LW, Green T, Griffiths AM, Kistner EO, Murtha MT, Regueiro MD, Rotter JI, Schumm LP, Steinhart AH, Targan SR, Xavier RJ, Libioulle C, Sandor C, Lathrop M, Belaiche J, Dewit O, Gut I, Heath S, Laukens D, Mni M, Rutgeerts P, Van Gossum A, Zelenika D, Franchimont D, Hugot JP, de Vos M, Vermeire S, Louis E, Cardon LR, Anderson CA, Drummond H, Nimmo E, Ahmad T, Prescott NJ, Onnie CM, Fisher SA, Marchini J, Ghori J, Bumpstead S, Gwilliam R, Tremelling M, Deloukas P, Mansfield J, Jewell D, Satsangi J, Mathew CG, Parkes M, Georges M, Daly MJ. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet 40: 955– 962, 2008 - PMC - PubMed
-
- Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 369: 1641– 1657, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

