Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews
- PMID: 22461129
- PMCID: PMC3396310
- DOI: 10.1093/ije/dys041
Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews
Abstract
Background: Many meta-analyses contain only a small number of studies, which makes it difficult to estimate the extent of between-study heterogeneity. Bayesian meta-analysis allows incorporation of external evidence on heterogeneity, and offers advantages over conventional random-effects meta-analysis. To assist in this, we provide empirical evidence on the likely extent of heterogeneity in particular areas of health care.
Methods: Our analyses included 14 886 meta-analyses from the Cochrane Database of Systematic Reviews. We classified each meta-analysis according to the type of outcome, type of intervention comparison and medical specialty. By modelling the study data from all meta-analyses simultaneously, using the log odds ratio scale, we investigated the impact of meta-analysis characteristics on the underlying between-study heterogeneity variance. Predictive distributions were obtained for the heterogeneity expected in future meta-analyses.
Results: Between-study heterogeneity variances for meta-analyses in which the outcome was all-cause mortality were found to be on average 17% (95% CI 10-26) of variances for other outcomes. In meta-analyses comparing two active pharmacological interventions, heterogeneity was on average 75% (95% CI 58-95) of variances for non-pharmacological interventions. Meta-analysis size was found to have only a small effect on heterogeneity. Predictive distributions are presented for nine different settings, defined by type of outcome and type of intervention comparison. For example, for a planned meta-analysis comparing a pharmacological intervention against placebo or control with a subjectively measured outcome, the predictive distribution for heterogeneity is a log-normal (-2.13, 1.58(2)) distribution, which has a median value of 0.12. In an example of meta-analysis of six studies, incorporating external evidence led to a smaller heterogeneity estimate and a narrower confidence interval for the combined intervention effect.
Conclusions: Meta-analysis characteristics were strongly associated with the degree of between-study heterogeneity, and predictive distributions for heterogeneity differed substantially across settings. The informative priors provided will be very beneficial in future meta-analyses including few studies.
Figures
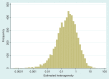
 ), plotted on log scale
), plotted on log scale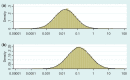
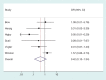
References
-
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7:177–88. - PubMed
-
- Jackson D, Bowden J, Baker R. How does the DerSimonian and Laird procedure for random effects meta-analysis compare with its more efficient but harder to compute counterparts? J Stat Plan Infer. 2010;140:961–70.
-
- Smith TC, Spiegelhalter DJ, Thomas A. Bayesian approaches to random-effects meta-analysis: a comparative study. Stat Med. 1995;14:2685–99. - PubMed
-
- Sutton AJ, Abrams KR. Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res. 2001;10:277–303. - PubMed
Publication types
MeSH terms
Grants and funding
- RG/08/014/24067/BHF_/British Heart Foundation/United Kingdom
- G0901530/MRC_/Medical Research Council/United Kingdom
- MC_U105285807/MRC_/Medical Research Council/United Kingdom
- U.1052.00.011.00003.01/MRC_/Medical Research Council/United Kingdom
- MC_U105260792/MRC_/Medical Research Council/United Kingdom

