Ski inhibits TGF-β/phospho-Smad3 signaling and accelerates hypertrophic differentiation in chondrocytes
- PMID: 22461172
- PMCID: PMC3324639
- DOI: 10.1002/jcb.24089
Ski inhibits TGF-β/phospho-Smad3 signaling and accelerates hypertrophic differentiation in chondrocytes
Abstract
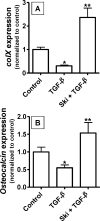
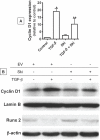
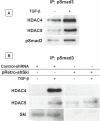
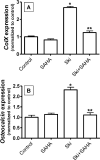
Since transforming growing factor-β (TGF-β)/Smad signaling inhibits chondrocyte maturation, endogenous negative regulators of TGF-β signaling are likely also important regulators of the chondrocyte differentiation process. One such negative regulator, Ski, is an oncoprotein that is known to inhibit TGF-β/Smad3 signaling via its interaction with phospho-Smad3 and recruitment of histone deacetylases (HDACs) to the DNA binding complex. Based on this, we hypothesized that Ski inhibits TGF-β signaling and accelerates maturation in chondrocytes via recruitment of HDACs to transcriptional complexes containing Smads. We tested this hypothesis in chick upper sternal chondrocytes (USCs), where gain and loss of Ski expression experiments were performed. Over-expression of Ski not only reversed the inhibitory effect of TGF-β on the expression of hypertrophic marker genes such as type X collagen (colX) and osteocalcin, it induced these genes basally as well. Conversely, knockdown of Ski by RNA interference led to a reduction of colX and osteocalcin expression under basal conditions. Furthermore, Ski blocked TGF-β induction of cyclinD1 and caused a basal up-regulation of Runx2, consistent with the observed acceleration of hypertrophy. Regarding mechanism, not only does Ski associate with phospho-Smad2 and 3, but its association with phospho-Smad3 is required for recruitment of HDAC4 and 5. Implicating this recruitment of HDACs in the phenotypic effects of Ski in chondrocytes, the HDAC inhibitor SAHA reversed the up-regulation of colX and osteocalcin in Ski over-expressing cells. These results suggest that inhibition of TGF-β signaling by Ski, which involves its association with phospho-Smad3 and recruitment of HDAC4 and 5, leads to accelerated chondrocyte differentiation.
Copyright © 2012 Wiley Periodicals, Inc.
Figures



Similar articles
-
Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation.J Bone Miner Res. 2006 Jan;21(1):4-16. doi: 10.1359/JBMR.050911. Epub 2005 Sep 19. J Bone Miner Res. 2006. PMID: 16355269 Free PMC article.
-
Differential role of Sloan-Kettering Institute (Ski) protein in Nodal and transforming growth factor-beta (TGF-β)-induced Smad signaling in prostate cancer cells.Carcinogenesis. 2012 Nov;33(11):2054-64. doi: 10.1093/carcin/bgs252. Epub 2012 Jul 27. Carcinogenesis. 2012. PMID: 22843506 Free PMC article.
-
Smad2 and 3 mediate transforming growth factor-beta1-induced inhibition of chondrocyte maturation.Endocrinology. 2000 Dec;141(12):4728-35. doi: 10.1210/endo.141.12.7848. Endocrinology. 2000. PMID: 11108288
-
Smad-Runx interactions during chondrocyte maturation.J Bone Joint Surg Am. 2001;83-A Suppl 1(Pt 1):S15-22. J Bone Joint Surg Am. 2001. PMID: 11263661 Review.
-
Repression of TGF-beta signaling by the oncogenic protein SKI in human melanomas: consequences for proliferation, survival, and metastasis.Oncogene. 2003 May 19;22(20):3123-9. doi: 10.1038/sj.onc.1206452. Oncogene. 2003. PMID: 12793438 Review.
Cited by
-
In-frame mutations in exon 1 of SKI cause dominant Shprintzen-Goldberg syndrome.Am J Hum Genet. 2012 Nov 2;91(5):950-7. doi: 10.1016/j.ajhg.2012.10.002. Epub 2012 Oct 25. Am J Hum Genet. 2012. PMID: 23103230 Free PMC article.
-
Integrative Analysis Reveals the Diverse Effects of 3D Stiffness upon Stem Cell Fate.Int J Mol Sci. 2023 May 26;24(11):9311. doi: 10.3390/ijms24119311. Int J Mol Sci. 2023. PMID: 37298263 Free PMC article.
-
Smad2 and Smad3 Regulate Chondrocyte Proliferation and Differentiation in the Growth Plate.PLoS Genet. 2016 Oct 14;12(10):e1006352. doi: 10.1371/journal.pgen.1006352. eCollection 2016 Oct. PLoS Genet. 2016. PMID: 27741240 Free PMC article.
-
c-Ski activates cancer-associated fibroblasts to regulate breast cancer cell invasion.Mol Oncol. 2013 Dec;7(6):1116-28. doi: 10.1016/j.molonc.2013.08.007. Epub 2013 Aug 27. Mol Oncol. 2013. PMID: 24011664 Free PMC article.
-
The roles and regulatory mechanisms of TGF-β and BMP signaling in bone and cartilage development, homeostasis and disease.Cell Res. 2024 Feb;34(2):101-123. doi: 10.1038/s41422-023-00918-9. Epub 2024 Jan 24. Cell Res. 2024. PMID: 38267638 Free PMC article. Review.
References
-
- Akiyoshi S, Inoue H, Hanai J, Kusanagi K, Nemoto N, Miyazono K, Kawabata M. c-Ski acts as a transcriptional co-repressor in transforming growth factor-beta signaling through interaction with smads. Journal of Biological Chemistry. 1999;274:35269–35277. - PubMed
-
- Ballock RT, Heydemann A, Wakefield LM, Flanders KC, Roberts AB, Sporn MB. TGF-beta1 prevents hypertrophy of epiphyseal chondrocytes: Regulation of gene expression for cartilage matrix proteins and metalloproteases. Developmental Biology. 1993;158:414–429. - PubMed
-
- Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr.Rev. 2003;24:218–235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

