HER3 is required for HER2-induced preneoplastic changes to the breast epithelium and tumor formation
- PMID: 22461506
- PMCID: PMC3693553
- DOI: 10.1158/0008-5472.CAN-11-3594
HER3 is required for HER2-induced preneoplastic changes to the breast epithelium and tumor formation
Abstract
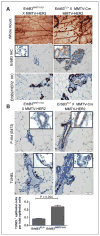
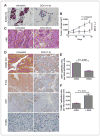
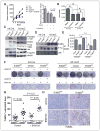
Increasing evidence suggests that HER2-amplified breast cancer cells use HER3/ErbB3 to drive therapeutic resistance to HER2 inhibitors. However, the role of ErbB3 in the earliest events of breast epithelial transformation remains unknown. Using mouse mammary specific models of Cre-mediated ErbB3 ablation, we show that ErbB3 loss prevents the progressive transformation of HER2-overexpressing mammary epithelium. Decreased proliferation and increased apoptosis were seen in MMTV-HER2 and MMTV-Neu mammary glands lacking ErbB3, thus inhibiting premalignant HER2-induced hyperplasia. Using a transgenic model in which HER2 and Cre are expressed from a single polycistronic transcript, we showed that palpable tumor penetrance decreased from 93.3% to 6.7% upon ErbB3 ablation. Penetrance of ductal carcinomas in situ was also decreased. In addition, loss of ErbB3 impaired Akt and p44/42 phosphorylation in preneoplastic HER2-overexpressing mammary glands and in tumors, decreased growth of preexisting HER2-overexpressing tumors, and improved tumor response to the HER2 tyrosine kinase inhibitor lapatinib. These events were rescued by reexpression of ErbB3, but were only partially rescued by ErbB36F, an ErbB3 mutant harboring six tyrosine-to-phenylalanine mutations that block its interaction with phosphatidyl inositol 3-kinase. Taken together, our findings suggest that ErbB3 promotes HER2-induced changes in the breast epithelium before, during, and after tumor formation. These results may have important translational implications for the treatment and prevention of HER2-amplified breast tumors through ErbB3 inhibition.
©2012 AACR.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

