Effects of sevelamer on HbA1c, inflammation, and advanced glycation end products in diabetic kidney disease
- PMID: 22461535
- PMCID: PMC3362316
- DOI: 10.2215/CJN.12891211
Effects of sevelamer on HbA1c, inflammation, and advanced glycation end products in diabetic kidney disease
Abstract
Background and objectives: Increased inflammation and oxidative stress may be caused by proteins and lipids modified by cytotoxic advanced glycation end products (AGEs) in food. Restricting food containing elevated AGEs improves these risk factors in diabetic CKD. Because diet adherence can be problematic, this study aimed to remove cytotoxic AGEs from food already ingested and to determine whether sevelamer carbonate sequesters cytotoxic AGEs in the gut, preventing their uptake and thereby reducing AGE-induced abnormalities.
Design, setting, participants, & measurements: This single-center, randomized, 2-month, open-label, intention-to-treat, crossover study compared sevelamer carbonate with calcium carbonate treatment in stage 2-4 diabetic CKD. Participants received 2 months of treatment with one drug, had a 1-week washout, and then received the opposite drug for 2 months.
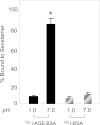
Results: Sevelamer carbonate reduced HbA1c, serum methylglyoxal, serum (ε)N-carboxymethyl-lysine, triglycerides, and 8-isoprostanes. Total cholesterol and fibroblast growth factor 23 were reduced by sevelamer carbonate, relative to calcium carbonate. AGE receptor 1 and sirtuin 1 mRNA were increased and PMNC TNFα levels were decreased by sevelamer carbonate, but not calcium carbonate. Medications and caloric and AGE intake remained unchanged. Sevelamer carbonate reversibly bound AGE-BSA at intestinal, but not stomach, pH.
Conclusions: Sevelamer carbonate significantly reduces HbA1c, fibroblast growth factor 23, lipids, and markers of inflammation and oxidative stress, and markedly increases antioxidant markers, independently of phosphorus in patients with diabetes and early kidney disease. These novel actions of sevelamer carbonate on metabolic and inflammatory abnormalities in type 2 diabetes mellitus may affect progression of early diabetic CKD.
Trial registration: ClinicalTrials.gov NCT01493050.
Figures

References
-
- Bennett WL, Wilson LM, Bolen S, Maruthur N, Singh S, Chatterjee R, Marinopoulos SS, Puhan MA, Ranasinghe P, Nicholson WK, Block L, Odelola O, Dalal DS, Ogbeche GE, Chandrasekhar A, Hutfless S, Bass EB, Segal JB: Oral diabetes medications for adults with type 2 diabetes: An update. In: Comparative Effectiveness Review, No. 27, Rockville, MD, Agency for Healthcare Research and Quality, 2011 - PubMed
-
- Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A: Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev 89: 27–71, 2009 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

