P2X7 receptor-mediated scavenger activity of mononuclear phagocytes toward non-opsonized particles and apoptotic cells is inhibited by serum glycoproteins but remains active in cerebrospinal fluid
- PMID: 22461619
- PMCID: PMC3366774
- DOI: 10.1074/jbc.M112.340885
P2X7 receptor-mediated scavenger activity of mononuclear phagocytes toward non-opsonized particles and apoptotic cells is inhibited by serum glycoproteins but remains active in cerebrospinal fluid
Abstract
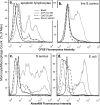
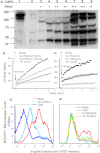
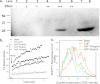
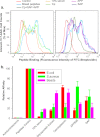
Rapid phagocytosis of non-opsonized particles including apoptotic cells is an important process that involves direct recognition of the target by multiple scavenger receptors including P2X7 on the phagocyte surface. Using a real-time phagocytosis assay, we studied the effect of serum proteins on this phagocytic process. Inclusion of 1-5% serum completely abolished phagocytosis of non-opsonized YG beads by human monocytes. Inhibition was reversed by pretreatment of serum with 1-10 mM tetraethylenepentamine, a copper/zinc chelator. Inhibitory proteins from the serum were determined as negatively charged glycoproteins (pI < 6) with molecular masses between 100 and 300 kDa. A glycoprotein-rich inhibitory fraction of serum not only abolished YG bead uptake but also inhibited phagocytosis of apoptotic lymphocytes or neuronal cells by human monocyte-derived macrophages. Three copper- and/or zinc-containing serum glycoproteins, ceruloplasmin, serum amyloid P-component, and amyloid precursor protein, were identified, and the purified proteins were shown to inhibit the phagocytosis of beads by monocytes as well as phagocytosis of apoptotic neuronal cells by macrophages. Human adult cerebrospinal fluid, which contains very little glycoprotein, had no inhibitory effect on phagocytosis of either beads or apoptotic cells. These data suggest for the first time that metal-interacting glycoproteins present within serum are able to inhibit the scavenger activity of mononuclear phagocytes toward insoluble debris and apoptotic cells.
Figures


References
-
- Cline M. J., Lehrer R. I. (1968) Phagocytosis by human monocytes. Blood 32, 423–435 - PubMed
-
- Ravichandran K. S. (2003) “Recruitment signals” from apoptotic cells. Invitation to a quiet meal. Cell 113, 817–820 - PubMed
-
- Ravichandran K. S., Lorenz U. (2007) Engulfment of apoptotic cells. Signals for a good meal. Nat. Rev. Immunol. 7, 964–974 - PubMed
-
- Park D., Tosello-Trampont A. C., Elliott M. R., Lu M., Haney L. B., Ma Z., Klibanov A. L., Mandell J. W., Ravichandran K. S. (2007) BAI-1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450, 430–434 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

