Beyond PSA: the next generation of prostate cancer biomarkers
- PMID: 22461644
- PMCID: PMC3799996
- DOI: 10.1126/scitranslmed.3003180
Beyond PSA: the next generation of prostate cancer biomarkers
Abstract
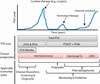
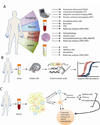
Since the introduction of serum prostate-specific antigen (PSA) screening 25 years ago, prostate cancer diagnosis and management have been guided by this biomarker. Yet, PSA has proven controversial as a screening assay owing to several inherent limitations. The next wave of prostate cancer biomarkers has emerged, introducing new assays in serum and urine that may supplement or, in time, replace PSA because of their higher cancer specificity. This expanding universe of biomarkers has been facilitated, in large part, by new genomic technologies that have enabled an unbiased look at cancer biology. Such efforts have produced several notable success stories that involve rapidly moving biomarkers from the bench to the clinic. However, biomarker research has centered on disease diagnostics, rather than prognosis and prediction, which would address disease management. The development of biomarkers to stratify risk of prostate cancer aggressiveness at the time of screening remains the greatest unmet clinical need in prostate cancer. We review the current state of prostate cancer biomarker research, including the PSA revolution, its impact on early cancer detection, the recent advances in biomarker discovery, and the future efforts that promise to improve clinical management of this disease.
Figures

References
-
- Sawyers CL. The cancer biomarker problem. Nature. 2008;452:548–552. - PubMed
-
- Oakman C, Santarpia L, Di Leo A. Breast cancer assessment tools and optimizing adjuvant therapy. Nat Rev Clin Oncol. 2010;7:725–732. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. - PubMed
-
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. - PMC - PubMed
-
- Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Janne PA, Costa DB, Varella-Garcia M, Kim WH, Lynch TJ, Fidias P, Stubbs H, Engelman JA, Sequist LV, Tan W, Gandhi L, Mino-Kenudson M, Wei GC, Shreeve SM, Ratain MJ, Settleman J, Christensen JG, Haber DA, Wilner K, Salgia R, Shapiro GI, Clark JW, Iafrate AJ. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

