Intracellular heat shock protein-70 negatively regulates TLR4 signaling in the newborn intestinal epithelium
- PMID: 22461698
- PMCID: PMC3331906
- DOI: 10.4049/jimmunol.1103114
Intracellular heat shock protein-70 negatively regulates TLR4 signaling in the newborn intestinal epithelium
Abstract
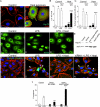
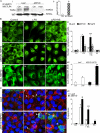
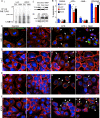
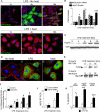
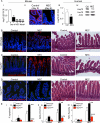
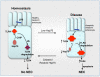
Necrotizing enterocolitis (NEC) is the leading cause of gastrointestinal-related mortality in premature infants, and it develops under conditions of exaggerated TLR4 signaling in the newborn intestinal epithelium. Because NEC does not develop spontaneously, despite the presence of seemingly tonic stimulation of intestinal TLR4, we hypothesized that mechanisms must exist to constrain TLR4 signaling that become diminished during NEC pathogenesis and focused on the intracellular stress response protein and chaperone heat shock protein-70 (Hsp70). We demonstrate that the induction of intracellular Hsp70 in enterocytes dramatically reduced TLR4 signaling, as assessed by LPS-induced NF-κB translocation, cytokine expression, and apoptosis. These findings were confirmed in vivo, using mice that either globally lacked Hsp70 or overexpressed Hsp70 within the intestinal epithelium. TLR4 activation itself significantly increased Hsp70 expression in enterocytes, which provided a mechanism of autoinhibition of TLR4 signaling in enterocytes. In seeking to define the mechanisms involved, intracellular Hsp70-mediated inhibition of TLR4 signaling required both its substrate-binding EEVD domain and association with the cochaperone CHIP, resulting in ubiquitination and proteasomal degradation of TLR4. The expression of Hsp70 in the intestinal epithelium was significantly decreased in murine and human NEC compared with healthy controls, suggesting that loss of Hsp70 protection from TLR4 could lead to NEC. In support of this, intestinal Hsp70 overexpression in mice and pharmacologic upregulation of Hsp70 reversed TLR4-induced cytokines and enterocyte apoptosis, as well as prevented and treated experimental NEC. Thus, a novel TLR4 regulatory pathway exists within the newborn gut involving Hsp70 that may be pharmacologically activated to limit NEC severity.
Figures



References
-
- Leaphart CL, Cavallo JC, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J. Immunol. 2007;179:4808–4820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

