Monoclonal antibodies to meningococcal factor H binding protein with overlapping epitopes and discordant functional activity
- PMID: 22461909
- PMCID: PMC3312907
- DOI: 10.1371/journal.pone.0034272
Monoclonal antibodies to meningococcal factor H binding protein with overlapping epitopes and discordant functional activity
Abstract
Background: Meningococcal factor H binding protein (fHbp) is a promising vaccine candidate. Anti-fHbp antibodies can bind to meningococci and elicit complement-mediated bactericidal activity directly. The antibodies also can block binding of the human complement down-regulator, factor H (fH). Without bound fH, the organism would be expected to have increased susceptibility to bacteriolysis. Here we describe bactericidal activity of two anti-fHbp mAbs with overlapping epitopes in relation to their different effects on fH binding and bactericidal activity.
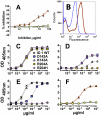
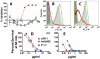
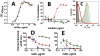
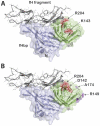
Methods and principal findings: Both mAbs recognized prevalent fHbp sequence variants in variant group 1. Using yeast display and site-specific mutagenesis, binding of one of the mAbs (JAR 1, IgG3) to fHbp was eliminated by a single amino acid substitution, R204A, and was decreased by K143A but not by R204H or D142A. The JAR 1 epitope overlapped that of previously described mAb (mAb502, IgG2a) whose binding to fHbp was eliminated by R204A or R204H substitutions, and was decreased by D142A but not by K143A. Although JAR 1 and mAb502 appeared to have overlapping epitopes, only JAR 1 inhibited binding of fH to fHbp and had human complement-mediated bactericidal activity. mAb502 enhanced fH binding and lacked human complement-mediated bactericidal activity. To control for confounding effects of different mouse IgG subclasses on complement activation, we created chimeric mAbs in which the mouse mAb502 or JAR 1 paratopes were paired with human IgG1 constant regions. While both chimeric mAbs showed similar binding to fHbp, only JAR 1, which inhibited fH binding, had human complement-mediated bactericidal activity.
Conclusions: The lack of human complement-mediated bactericidal activity by anti-fHbp mAb502 appeared to result from an inability to inhibit binding of fH. These results underscore the importance of inhibition of fH binding for anti-fHbp mAb bactericidal activity.
Conflict of interest statement
Figures

Similar articles
-
Cocrystal structure of meningococcal factor H binding protein variant 3 reveals a new crossprotective epitope recognized by human mAb 1E6.FASEB J. 2019 Nov;33(11):12099-12111. doi: 10.1096/fj.201900374R. Epub 2019 Oct 5. FASEB J. 2019. PMID: 31442074 Free PMC article.
-
Complement-mediated bactericidal activity of anti-factor H binding protein monoclonal antibodies against the meningococcus relies upon blocking factor H binding.Infect Immun. 2011 Sep;79(9):3751-9. doi: 10.1128/IAI.05182-11. Epub 2011 Jun 27. Infect Immun. 2011. PMID: 21708990 Free PMC article.
-
Combined roles of human IgG subclass, alternative complement pathway activation, and epitope density in the bactericidal activity of antibodies to meningococcal factor h binding protein.Infect Immun. 2012 Jan;80(1):187-94. doi: 10.1128/IAI.05956-11. Epub 2011 Nov 7. Infect Immun. 2012. PMID: 22064712 Free PMC article.
-
Does binding of complement factor H to the meningococcal vaccine antigen, factor H binding protein, decrease protective serum antibody responses?Clin Vaccine Immunol. 2013 Aug;20(8):1099-107. doi: 10.1128/CVI.00260-13. Epub 2013 Jun 5. Clin Vaccine Immunol. 2013. PMID: 23740919 Free PMC article. Review.
-
Meningococcal factor H binding protein as immune evasion factor and vaccine antigen.FEBS Lett. 2020 Aug;594(16):2657-2669. doi: 10.1002/1873-3468.13793. Epub 2020 May 12. FEBS Lett. 2020. PMID: 32298465 Review.
Cited by
-
Human protective response induced by meningococcus B vaccine is mediated by the synergy of multiple bactericidal epitopes.Sci Rep. 2018 Feb 27;8(1):3700. doi: 10.1038/s41598-018-22057-7. Sci Rep. 2018. PMID: 29487324 Free PMC article.
-
Two human antibodies to a meningococcal serogroup B vaccine antigen enhance binding of complement Factor H by stabilizing the Factor H binding site.PLoS Pathog. 2021 Jun 14;17(6):e1009655. doi: 10.1371/journal.ppat.1009655. eCollection 2021 Jun. PLoS Pathog. 2021. PMID: 34125873 Free PMC article.
-
Defining a protective epitope on factor H binding protein, a key meningococcal virulence factor and vaccine antigen.Proc Natl Acad Sci U S A. 2013 Feb 26;110(9):3304-9. doi: 10.1073/pnas.1222845110. Epub 2013 Feb 8. Proc Natl Acad Sci U S A. 2013. PMID: 23396847 Free PMC article.
-
Cocrystal structure of meningococcal factor H binding protein variant 3 reveals a new crossprotective epitope recognized by human mAb 1E6.FASEB J. 2019 Nov;33(11):12099-12111. doi: 10.1096/fj.201900374R. Epub 2019 Oct 5. FASEB J. 2019. PMID: 31442074 Free PMC article.
-
High throughput functional epitope mapping: revisiting phage display platform to scan target antigen surface.MAbs. 2014;6(6):1368-76. doi: 10.4161/mabs.36144. MAbs. 2014. PMID: 25484050 Free PMC article. Review.
References
-
- Finne J, Bitter-Suermann D, Goridis C, Finne U. An IgG monoclonal antibody to group B meningococci cross-reacts with developmentally regulated polysialic acid units of glycoproteins in neural and extraneural tissues. J Immunol. 1987;138:4402–4407. - PubMed
-
- Finne J, Leinonen M, Makela PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983;2:355–357. - PubMed
-
- Jennings HJ, Roy R, Gamian A. Induction of meningococcal group B polysaccharide-specific IgG antibodies in mice by using an N-propionylated B polysaccharide-tetanus toxoid conjugate vaccine. J Immunol. 1986;137:1708–1713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

