TNF-α is involved in the abnormal thymocyte migration during experimental Trypanosoma cruzi infection and favors the export of immature cells
- PMID: 22461911
- PMCID: PMC3312912
- DOI: 10.1371/journal.pone.0034360
TNF-α is involved in the abnormal thymocyte migration during experimental Trypanosoma cruzi infection and favors the export of immature cells
Abstract
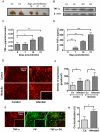
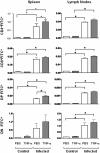
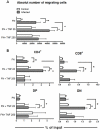
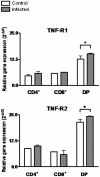
Previous studies revealed a significant production of inflammatory cytokines together with severe thymic atrophy and thymocyte migratory disturbances during experimental Chagas disease. Migratory activity of thymocytes and mature T cells seem to be finely tuned by cytokines, chemokines and extracellular matrix (ECM) components. Systemic TNF-α is enhanced during infection and appears to be crucial in the response against the parasite. However, it also seems to be involved in disease pathology, since it is implicated in the arrival of T cells to effector sites, including the myocardium. Herein, we analyzed the role of TNF-α in the migratory activity of thymocytes in Trypanosoma cruzi (T. cruzi) acutely-infected mice. We found increased expression and deposition of TNF-α in the thymus of infected animals compared to controls, accompanied by increased co-localization of fibronectin, a cell migration-related ECM molecule, whose contents in the thymus of infected mice is also augmented. In-vivo studies showed an enhanced export of thymocytes in T. cruzi-infected mice, as ascertained by intrathymic injection of FITC alone or in combination with TNF-α. The increase of immature CD4(+)CD8(+) T cells in secondary lymphoid organs was even more clear-cut when TNF-α was co-injected with FITC. Ex-vivo transmigration assays also revealed higher number of migrating cells when TNF-α was added onto fibronectin lattices, with higher input of all thymocyte subsets, including immature CD4(+)CD8(+). Infected animals also exhibit enhanced levels of expression of both mRNA TNF-α receptors in the CD4(+)CD8(+) subpopulation. Our findings suggest that in T. cruzi acute infection, when TNF-α is complexed with fibronectin, it favours the altered migration of thymocytes, promoting the release of mature and immature T cells to different compartments of the immune system. Conceptually, this work reinforces the notion that thymocyte migration is a multivectorial biological event in health and disease, and that TNF-α is a further player in the process.
Conflict of interest statement
Figures
References
-
- Leite-de-Moraes MC, Hontebeyrie-Joskowicz M, Leboulenger F, Savino W, Dardenne M, et al. Studies on the thymus in Chagas' disease. II. Thymocyte subset fluctuations in Trypanosoma cruzi-infected mice: relationship to stress. Scand J Immunol. 1991;33:267–275. - PubMed
-
- Pérez AR, Roggero E, Nicora A, Palazzi J, Besedovsky HO, et al. Thymus atrophy during Trypanosoma cruzi infection is caused by an immuno-endocrine imbalance. Brain Behav Immun. 2007;21:890–900. - PubMed
-
- Cotta-de-Almeida V, Bonomo A, Mendes-da-Cruz DA, Riederer I, De Meis J, et al. Trypanosoma cruzi infection modulates intrathymic contents of extracellular matrix ligands and receptors and alters thymocyte migration. Eur J Immunol. 2003;33:2439–2448. - PubMed
-
- Mendes-da-Cruz DA, Silva JS, Cotta-de-Almeida V, Savino W. Altered thymocyte migration during experimental acute Trypanosoma cruzi infection: combined role of fibronectin and the chemokines CXCL12 and CCL4. Eur J Immunol. 2006;36:1486–1493. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

