A broadly cross-reactive monoclonal antibody against an epitope on the n-terminus of meningococcal fHbp
- PMID: 22461972
- PMCID: PMC3314305
- DOI: 10.1038/srep00341
A broadly cross-reactive monoclonal antibody against an epitope on the n-terminus of meningococcal fHbp
Erratum in
- Sci Rep. 2013 Mar 20;3. doi:10.1038/srep01499
Abstract
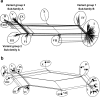
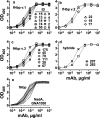
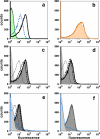
Meningococcal factor H binding protein (fHbp) is an important vaccine antigen for prevention of disease caused by capsular group B strains. The protein has been sub-classified into three variant groups. Most anti-fHbp antibodies are variant group-specific and recognize epitopes on the C-terminal domain. We report a murine IgG1 mAb, JAR 41, which broadly cross-reacted with fHbp sequence variants from all variant groups. The mAb bound to the surface of live meningococci with fHbp from each of the three variant groups. In combination with second non-bactericidal anti-fHbp mAbs, JAR 41 elicited complement-mediated bactericidal activity in vitro, and augmented passive protection against meningococcal bacteremia in human fH transgenic rats. The epitope was located on a conserved region of the N-terminal portion of the fHbp molecule opposite that of fH contact residues. The data underscore the importance of broadly cross-reactive, surface-exposed epitopes on the N-terminal domain in the design of protective fHbp vaccines.
Conflict of interest statement
D.M.G. holds a paid consultancy with Novartis Vaccines and Diagnostics. D.M.G. R.P. and D.M.V. are inventors on patents or patent applications in the area of meningococcal B vaccines. D.C.R. report no conflicts of interest.
Figures
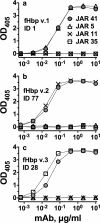
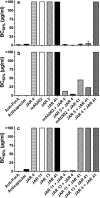
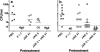
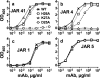
 (Xs with solid line), wild-type fHbp ID 1;
(Xs with solid line), wild-type fHbp ID 1;  (Open squares with dotted line), H26A;
(Open squares with dotted line), H26A;  (Open triangles with dashed line), K27A;
(Open triangles with dashed line), K27A;  (Open circles with solid line), D25A. Panel a, JAR 41. Panel b, JAR 4. Panel c, JAR 1. Panel d, JAR 5.
(Open circles with solid line), D25A. Panel a, JAR 41. Panel b, JAR 4. Panel c, JAR 1. Panel d, JAR 5.Similar articles
-
Monoclonal antibodies to meningococcal factor H binding protein with overlapping epitopes and discordant functional activity.PLoS One. 2012;7(3):e34272. doi: 10.1371/journal.pone.0034272. Epub 2012 Mar 26. PLoS One. 2012. PMID: 22461909 Free PMC article.
-
A region of the N-terminal domain of meningococcal factor H-binding protein that elicits bactericidal antibody across antigenic variant groups.Mol Immunol. 2009 May;46(8-9):1647-53. doi: 10.1016/j.molimm.2009.02.021. Epub 2009 Mar 14. Mol Immunol. 2009. PMID: 19286260 Free PMC article.
-
Two cross-reactive monoclonal antibodies recognize overlapping epitopes on Neisseria meningitidis factor H binding protein but have different functional properties.FASEB J. 2014 Apr;28(4):1644-53. doi: 10.1096/fj.13-239012. Epub 2013 Dec 26. FASEB J. 2014. PMID: 24371123
-
Meningococcal serogroup B vaccines: Estimating breadth of coverage.Hum Vaccin Immunother. 2017 Feb;13(2):255-265. doi: 10.1080/21645515.2017.1264750. Epub 2016 Dec 14. Hum Vaccin Immunother. 2017. PMID: 27960595 Free PMC article. Review.
-
Meningococcal factor H binding protein as immune evasion factor and vaccine antigen.FEBS Lett. 2020 Aug;594(16):2657-2669. doi: 10.1002/1873-3468.13793. Epub 2020 May 12. FEBS Lett. 2020. PMID: 32298465 Review.
Cited by
-
Defining a protective epitope on factor H binding protein, a key meningococcal virulence factor and vaccine antigen.Proc Natl Acad Sci U S A. 2013 Feb 26;110(9):3304-9. doi: 10.1073/pnas.1222845110. Epub 2013 Feb 8. Proc Natl Acad Sci U S A. 2013. PMID: 23396847 Free PMC article.
-
Binding of complement factor H to PorB3 and NspA enhances resistance of Neisseria meningitidis to anti-factor H binding protein bactericidal activity.Infect Immun. 2015 Apr;83(4):1536-45. doi: 10.1128/IAI.02984-14. Epub 2015 Feb 2. Infect Immun. 2015. PMID: 25644002 Free PMC article.
-
Bactericidal human monoclonal antibody 1B1 shows specificity for meningococcal factor H binding protein variant 2 and displaces human factor H.FASEB Bioadv. 2024 Jun 27;6(8):235-248. doi: 10.1096/fba.2023-00077. eCollection 2024 Aug. FASEB Bioadv. 2024. PMID: 39114449 Free PMC article.
-
4CMenB vaccine induces elite cross-protective human antibodies that compete with human factor H for binding to meningococcal fHbp.PLoS Pathog. 2020 Oct 2;16(10):e1008882. doi: 10.1371/journal.ppat.1008882. eCollection 2020 Oct. PLoS Pathog. 2020. PMID: 33007046 Free PMC article.
-
A Mutant Library Approach to Identify Improved Meningococcal Factor H Binding Protein Vaccine Antigens.PLoS One. 2015 Jun 9;10(6):e0128185. doi: 10.1371/journal.pone.0128185. eCollection 2015. PLoS One. 2015. PMID: 26057742 Free PMC article.
References
-
- Sadarangani M. & Pollard A. J. Serogroup B meningococcal vaccines-an unfinished story. Lancet Infect. Dis. 10, 112–124 (2010). - PubMed
-
- Snape M. D. et al. Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life: a randomized comparative trial. Pediatr. Infect. Dis. J. 29, e71–79 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

