Endogenously released ACh and exogenous nicotine differentially facilitate long-term potentiation induction in the hippocampal CA1 region of mice
- PMID: 22462479
- PMCID: PMC3348254
- DOI: 10.1111/j.1460-9568.2012.08056.x
Endogenously released ACh and exogenous nicotine differentially facilitate long-term potentiation induction in the hippocampal CA1 region of mice
Abstract
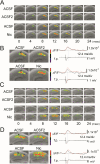
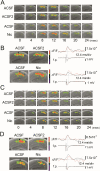
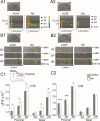
We examined the role of α7- and β2-containing nicotinic acetylcholine receptors (nAChRs) in the induction of long-term potentiation (LTP). Theta-burst stimulation (TBS), mimicking the brain's naturally occurring theta rhythm, induced robust LTP in hippocampal slices from α7 and β2 knockout mice. This suggests TBS is capable of inducing LTP without activation of α7- or β2-containing nAChRs. However, when weak TBS was applied, the modulatory effects of nicotinic receptors on LTP induction became visible. We showed that during weak TBS, activation of α7 nAChRs occurs by the release of ACh, contributing to LTP induction. Additionally, bath-application of nicotine activated β2-containing nAChRs to promote LTP induction. Despite predicted nicotine-induced desensitization, synaptically mediated activation of α7 nAChRs still occurs in the presence of nicotine and contributed to LTP induction. Optical recording of single-stimulation-evoked excitatory activity with a voltage-sensitive dye revealed enhanced excitatory activity in the presence of nicotine. This effect of nicotine was robust during high-frequency stimulation, and was accompanied by enhanced burst excitatory postsynaptic potentials. Nicotine-induced enhancement of excitatory activity was observed in slices from α7 knockout mice, but was absent in β2 knockout mice. These results suggest that the nicotine-induced enhancement of excitatory activity is mediated by β2-containing nAChRs, and is related to the nicotine-induced facilitation of LTP induction. Thus, our study demonstrates that the activation of α7- and β2-containing nAChRs differentially facilitates LTP induction via endogenously released ACh and exogenous nicotine, respectively, in the hippocampal CA1 region of mice.
© 2012 The Authors. European Journal of Neuroscience © 2012 Federation of European Neuroscience Societies and Blackwell Publishing Ltd.
Figures
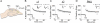





Similar articles
-
Nicotine-induced switch in the nicotinic cholinergic mechanisms of facilitation of long-term potentiation induction.Eur J Neurosci. 2005 Aug;22(4):845-60. doi: 10.1111/j.1460-9568.2005.04259.x. Eur J Neurosci. 2005. PMID: 16115208
-
Inactivation of alpha7 ACh receptors and activation of non-alpha7 ACh receptors both contribute to long term potentiation induction in the hippocampal CA1 region.Neurosci Lett. 2000 Jun 2;286(2):134-8. doi: 10.1016/s0304-3940(00)01076-4. Neurosci Lett. 2000. PMID: 10825655
-
In hippocampal oriens interneurons anti-Hebbian long-term potentiation requires cholinergic signaling via α7 nicotinic acetylcholine receptors.J Neurosci. 2013 Jan 16;33(3):1044-9. doi: 10.1523/JNEUROSCI.1070-12.2013. J Neurosci. 2013. PMID: 23325242 Free PMC article.
-
Heteromeric α7β2 Nicotinic Acetylcholine Receptors in the Brain.Trends Pharmacol Sci. 2016 Jul;37(7):562-574. doi: 10.1016/j.tips.2016.03.005. Epub 2016 May 11. Trends Pharmacol Sci. 2016. PMID: 27179601 Free PMC article. Review.
-
Exposure to nicotine and sensitization of nicotine-induced behaviors.Prog Neuropsychopharmacol Biol Psychiatry. 2007 Nov 15;31(8):1625-38. doi: 10.1016/j.pnpbp.2007.08.038. Epub 2007 Sep 1. Prog Neuropsychopharmacol Biol Psychiatry. 2007. PMID: 17936462 Free PMC article. Review.
Cited by
-
Gating of long-term potentiation by nicotinic acetylcholine receptors at the cerebellum input stage.PLoS One. 2013 May 31;8(5):e64828. doi: 10.1371/journal.pone.0064828. Print 2013. PLoS One. 2013. PMID: 23741401 Free PMC article.
-
Activation of α7 nAChR by PNU-282987 improves synaptic and cognitive functions through restoring the expression of synaptic-associated proteins and the CaM-CaMKII-CREB signaling pathway.Aging (Albany NY). 2020 Jan 6;12(1):543-570. doi: 10.18632/aging.102640. Epub 2020 Jan 6. Aging (Albany NY). 2020. PMID: 31905173 Free PMC article.
-
Neonatal nicotine exposure increases excitatory synaptic transmission and attenuates nicotine-stimulated GABA release in the adult rat hippocampus.Neuropharmacology. 2015 Jan;88:187-98. doi: 10.1016/j.neuropharm.2014.06.010. Epub 2014 Jun 17. Neuropharmacology. 2015. PMID: 24950455 Free PMC article.
-
How cigarette smoking may increase the risk of anxiety symptoms and anxiety disorders: a critical review of biological pathways.Brain Behav. 2013 May;3(3):302-26. doi: 10.1002/brb3.137. Epub 2013 Mar 26. Brain Behav. 2013. PMID: 23785661 Free PMC article.
-
Endogenous ACh suppresses LTD induction and nicotine relieves the suppression via different nicotinic ACh receptor subtypes in the mouse hippocampus.Life Sci. 2014 Aug 28;111(1-2):62-8. doi: 10.1016/j.lfs.2014.07.014. Epub 2014 Jul 19. Life Sci. 2014. PMID: 25046735 Free PMC article.
References
-
- Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptor alpha7 and alpha4beta2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol. 2001;86:3043–3055. - PubMed
-
- Alkondon M, Pereira EF, Albuquerque EX. alpha-bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res. 1998;810:257–263. - PubMed
-
- Alkondon M, Pereira EF, Almeida LE, Randall WR, Albuquerque EX. Nicotine at concentrations found in cigarette smokers activates and desensitizes nicotinic acetylcholine receptors in CA1 interneurons of rat hippocampus. Neuropharmacology. 2000;39:2726–2739. - PubMed
-
- Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther. 1997;283:1396–1411. - PubMed
-
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

