JS-K, a glutathione/glutathione S-transferase-activated nitric oxide releasing prodrug inhibits androgen receptor and WNT-signaling in prostate cancer cells
- PMID: 22462810
- PMCID: PMC3376035
- DOI: 10.1186/1471-2407-12-130
JS-K, a glutathione/glutathione S-transferase-activated nitric oxide releasing prodrug inhibits androgen receptor and WNT-signaling in prostate cancer cells
Abstract
Background: Nitric oxide (NO) and its oxidative reaction products have been repeatedly shown to block steroid receptor function via nitrosation of zinc finger structures in the DNA-binding domain (DBD). In consequence NO-donors could be of special interest for the treatment of deregulated androgen receptor(AR)-signaling in castration resistant prostate cancer (CRPC).
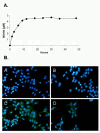
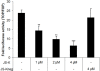
Methods: Prostate cancer (PCa) cells were treated with JS-K, a diazeniumdiolate derivate capable of generating large amounts of intracellular NO following activation by glutathione S-transferase. Generation of NO was determined indirectly by the detection of nitrate in tissue culture medium or by immunodetection of nitrotyrosine in the cytoplasm. Effects of JS-K on intracellular AR-levels were determined by western blotting. AR-dimerization was analyzed by mammalian two hybrid assay, nuclear translocation of the AR was visualized in PCa cells transfected with a green fluorescent AR-Eos fusion protein using fluorescence microscopy. Modulation of AR- and WNT-signalling by JS-K was investigated using reporter gene assays. Tumor cell proliferation following JS-K treatment was measured by MTT-Assay.
Results: The NO-releasing compound JS-K was shown to inhibit AR-mediated reporter gene activity in 22Rv1 CRPC cells. Inhibition of AR signaling was neither due to an inhibition of nuclear import nor to a reduction in AR-dimerization. In contrast to previously tested NO-donors, JS-K was able to reduce the intracellular concentration of functional AR. This could be attributed to the generation of extremely high intracellular levels of the free radical NO as demonstrated indirectly by high levels of nitrotyrosine in JS-K treated cells. Moreover, JS-K diminished WNT-signaling in AR-positive 22Rv1 cells. In line with these observations, castration resistant 22Rv1 cells were found to be more susceptible to the growth inhibitory effects of JS-K than the androgen dependent LNCaP which do not exhibit an active WNT-signaling pathway.
Conclusions: Our results suggest that small molecules able to inhibit WNT- and AR-signaling via NO-release represent a promising platform for the development of new compounds for the treatment of CRPC.
Figures
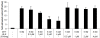
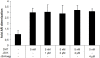



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

