Replacing conventional carbon nucleophiles with electrophiles: nickel-catalyzed reductive alkylation of aryl bromides and chlorides
- PMID: 22463689
- PMCID: PMC3324882
- DOI: 10.1021/ja301769r
Replacing conventional carbon nucleophiles with electrophiles: nickel-catalyzed reductive alkylation of aryl bromides and chlorides
Abstract
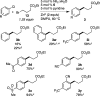
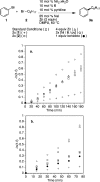
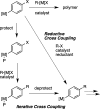
A general method is presented for the synthesis of alkylated arenes by the chemoselective combination of two electrophilic carbons. Under the optimized conditions, a variety of aryl and vinyl bromides are reductively coupled with alkyl bromides in high yields. Under similar conditions, activated aryl chlorides can also be coupled with bromoalkanes. The protocols are highly functional-group tolerant (-OH, -NHTs, -OAc, -OTs, -OTf, -COMe, -NHBoc, -NHCbz, -CN, -SO(2)Me), and the reactions are assembled on the benchtop with no special precautions to exclude air or moisture. The reaction displays different chemoselectivity than conventional cross-coupling reactions, such as the Suzuki-Miyaura, Stille, and Hiyama-Denmark reactions. Substrates bearing both an electrophilic and nucleophilic carbon result in selective coupling at the electrophilic carbon (R-X) and no reaction at the nucleophilic carbon (R-[M]) for organoboron (-Bpin), organotin (-SnMe(3)), and organosilicon (-SiMe(2)OH) containing organic halides (X-R-[M]). A Hammett study showed a linear correlation of σ and σ(-) parameters with the relative rate of reaction of substituted aryl bromides with bromoalkanes. The small ρ values for these correlations (1.2-1.7) indicate that oxidative addition of the bromoarene is not the turnover-frequency determining step. The rate of reaction has a positive dependence on the concentration of alkyl bromide and catalyst, no dependence upon the amount of zinc (reducing agent), and an inverse dependence upon aryl halide concentration. These results and studies with an organic reductant (TDAE) argue against the intermediacy of organozinc reagents.
© 2012 American Chemical Society
Figures



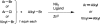



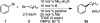

References
-
-
For reviews of the reactions used in process chemistry, see:
- Dugger R.; Ragan J.; Ripin D. Org. Process Res. Dev. 2005, 9, 253–258.
- Carey J.; Laffan D.; Thomson C.; Williams M. Org. Biomol. Chem. 2006, 4, 2337–2347. - PubMed
- Laird T. Org. Process Res. Dev. 2006, 10, 851–852.
-
-
-
The most commercially available nucleophilic carbon source used in cross-coupling today is the boronic acid. A search of Scifinder Scholar on 9/7/2011 found 5,484 commercially available substances of the general structure C*B(OH)2, where “C*” was any substituents off of carbon. Similar exhaustive searches for R–I and R–Br produced over 80,000 and 700,000 substances in 2010.
-
-
- Molander G. A.; Ellis N. Acc. Chem. Res. 2007, 40, 275–286. - PubMed
-
Carbon–boron bonds can be prepared directly from C–H bonds by transition metal catalysis:
- Mkhalid I. A. I.; Barnard J. H.; Marder T. B.; Murphy J. M.; Hartwig J. F. Chem. Rev. 2009, 110, 890–931. - PubMed
-
Preparation of aryl boronic acids from aryl halides can be accomplished by transition metal catalysis. For selected examples, see:
- Haddenham D.; Bailey C. L.; Vu C.; Nepomuceno G.; Eagon S.; Pasumansky L.; Singaram B. Tetrahedron 2011, 67, 576–583.
- Wilson D. A.; Wilson C. J.; Moldoveanu C.; Resmerita A.-M.; Corcoran P.; Hoang L. M.; Rosen B. M.; Percec V. J. Am. Chem. Soc. 2010, 132, 1800–1801. - PubMed
- Molander G. A.; Trice S. L. J.; Dreher S. D. J. Am. Chem. Soc. 2010, 132, 17701–17703. - PMC - PubMed
- Moldoveanu C.; Wilson D. A.; Wilson C. J.; Corcoran P.; Rosen B. M.; Percec V. Org. Lett. 2009, 11, 4974–4977. - PubMed
- Murata M.; Oyama T.; Watanabe S.; Masuda Y. J. Org. Chem. 1999, 65, 164–168. - PubMed
- Ishiyama T.; Murata M.; Miyaura N. J. Org. Chem. 1995, 60, 7508–7510.
-
For preparation of boronic acids from organolithiums or Grignards and trialkylborates, see:
- Brown H. C.; Rangaishenvi M. V. Tetrahedron Lett. 1990, 31, 7113–7114.
- Brown H. C.; Bhat N. G.; Srebnik M. Tetrahedron Lett. 1988, 29, 2631–2634.
- Brown H. C.; Cole T. E. Organometallics 1983, 2, 1316–1319.
- Matteson D. S.; Liedtke J. D. J. Am. Chem. Soc. 1965, 87, 1526–1531.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

