Home medicines reviews following acute coronary syndrome: study protocol for a randomized controlled trial
- PMID: 22463733
- PMCID: PMC3349589
- DOI: 10.1186/1745-6215-13-30
Home medicines reviews following acute coronary syndrome: study protocol for a randomized controlled trial
Abstract
Background: Despite continual improvements in the management of acute coronary syndromes, adherence to guideline-based medications remains suboptimal. We aim to improve adherence with guideline-based therapy following acute coronary syndrome using an existing service that is provided by specifically trained pharmacists, called a Home Medicines Review. We have made two minor adjustments to target the focus of the existing service including an acute coronary syndrome specific referral letter and a training package for the pharmacists providing the service.
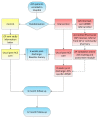
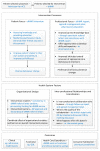
Methods/design: We will be conducting a randomized controlled trial to compare the directed home medicines review service to usual care following acute coronary syndromes. All patients aged 18 to 80 years and with a working diagnosis of acute coronary syndrome, who are admitted to two public, acute care hospitals, will be screened for enrolment into the trial. Exclusion criteria will include: not being discharged home, documented cognitive decline, non-Medicare eligibility, and presence of a terminal malignancy. Randomization concealment and sequence generation will occur through a centrally-monitored computer program. Patients randomized to the control group will receive usual post-discharge care. Patients randomized to receive the intervention will be offered usual post-discharge care and a directed home medicines review at two months post-discharge. The study endpoints will be six and twelve months post-discharge. The primary outcome will be the proportion of patients who are adherent to a complete, guideline-based medication regimen. Secondary outcomes will include hospital readmission rates, length of hospital stays, changes in quality of life, smoking cessation rates, cardiac rehabilitation completion rates, and mortality.
Discussion: As the trial is closely based on an existing service, any improvements observed should be highly translatable into regular practice. Possible limitations to the success of the trial intervention include general practitioner approval of the intervention, general practitioner acceptance of pharmacists' recommendations, and pharmacists' ability to make appropriate recommendations. A detailed monitoring process will detect any barriers to the success of the trial. Given that poor medication persistence following acute coronary syndrome is a worldwide problem, the findings of our study may have international implications for the care of this patient group.
Trial registration: Australian New Zealand Clinical Trials Registry ACTRN12611000452998.
Figures




References
-
- Peterson GM, Thompson A, Pulver LK, Robertson MB, Brieger D, Wai A, Tett SE, for the Dmacs Project Group. Management of acute coronary syndromes at hospital discharge: do targeted educational interventions improve practice quality? J Healthc Qual. 2011. doi:10.1111/j.1945-1474.2011.00137.x. - PubMed
-
- Airoldi F, Colombo A, Morici N, Latib A, Cosgrave J, Buellesfeld L, Bonizzoni E, Carlino M, Gerckens U, Godino C, Melzi G, Michev I, Montorfano M, Sangiorgi GM, Qasim A, Chieffo A, Briguori C, Grube E. Incidence and predictors of drug-eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation. 2007;116:745–754. doi: 10.1161/CIRCULATIONAHA.106.686048. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

