Glucagon-like peptides 1 and 2 and vasoactive intestinal peptide are neuroprotective on cultured and mast cell co-cultured rat myenteric neurons
- PMID: 22463807
- PMCID: PMC3352054
- DOI: 10.1186/1471-230X-12-30
Glucagon-like peptides 1 and 2 and vasoactive intestinal peptide are neuroprotective on cultured and mast cell co-cultured rat myenteric neurons
Abstract
Background: Neuropathy is believed to be a common feature of functional and inflammatory intestinal diseases. Vasoactive intestinal peptide (VIP) is an acknowledged neuroprotective agent in peripheral, including enteric, and central neurons. The proglucagon-like hormones glucagon-like peptide 1 and 2 (GLP1 and GLP2) belong to the secretin/glucagon/VIP superfamily of peptides and GLP1 and GLP2 receptors are expressed in enteric neurons. Possible neuroprotective effects of these peptides were investigated in the present study.
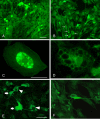
Methods: GLP1, GLP2 and VIP were added to cultured myenteric neurons from rat small intestine or to co-cultures of myenteric neurons and rat peritoneal mast cells. Receptor selectivity was tested by the simultaneous presence of a GLP1 receptor antagonist (exendin (9-39) amide) or a VIP receptor antagonist (hybrid of neurotensin 6-11 and VIP 7-28). Neuronal survival was examined using immunocytochemistry and cell counting.
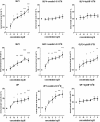
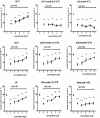
Results: GLP1, GLP2 and VIP significantly and concentration-dependently enhanced neuronal survival. In addition the peptides efficiently counteracted mast cell-induced neuronal cell death in a concentration-dependent manner. Exendin(9-39)amide reversed GLP1-induced neuroprotection while GLP2- and VIP-induced enhanced neuronal survival were unaffected. The VIP receptor antagonist reversed GLP1- and VIP-induced neuroprotection while the GLP2-induced effect on neuronal survival was unaffected.
Conclusions: By activating separate receptors VIP, GLP1 and GLP2 elicit neuroprotective effects on rat myenteric neurons cultured with or without mast cells. This implies a powerful therapeutic potential of these peptides in enteric neuropathies with a broad spectrum of applications from autoimmunity to functional disorders.
Figures
References
-
- Hammar J, Howell S, Bytzer P, Horowitz M, Talley NJ. Symptom clustering in subjects with and without diabetes mellitus: A population-based study of 15 000 Australian adults. Am J Gastroenterol. 2003;98:391–398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

