Amygdala hyperactivity and tonotopic shift after salicylate exposure
- PMID: 22464181
- PMCID: PMC5319430
- DOI: 10.1016/j.brainres.2012.03.016
Amygdala hyperactivity and tonotopic shift after salicylate exposure
Abstract
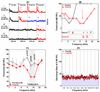
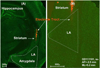
The amygdala, important in forming and storing memories of aversive events, is believed to play a major role in debilitating tinnitus and hyperacusis. To explore this hypothesis, we recorded from the lateral amygdala (LA) and auditory cortex (AC) before and after treating rats with a dose of salicylate that induces tinnitus and hyperacusis-like behavior. Salicylate unexpectedly increased the amplitude of the local field potential (LFP) in the LA making it hyperactive to sounds≥60 dB SPL. Frequency receptive fields (FRFs) of multiunit (MU) clusters in the LA were also dramatically altered by salicylate. Neuronal activity at frequencies below 10 kHz and above 20 kHz was depressed at low intensities, but was greatly enhanced for stimuli between 10 and 20 kHz (frequencies near the pitch of the salicylate-induced tinnitus in the rat). These frequency-dependent changes caused the FRF of many LA neurons to migrate towards 10-20 kHz thereby amplifying activity from this region. To determine if salicylate-induced changes restricted to the LA would remotely affect neural activity in the AC, we used a micropipette to infuse salicylate (20 μl, 2.8 mM) into the amygdala. Local delivery of salicylate to the amygdala significantly increased the amplitude of the LFP recorded in the AC and selectively enhanced the neuronal activity of AC neurons at the mid-frequencies (10-20 kHz), frequencies associated with the tinnitus pitch. Taken together, these results indicate that systemic salicylate treatment can induce hyperactivity and tonotopic shift in the amygdala and infusion of salicylate into the amygdala can profoundly enhance sound-evoked activity in AC, changes likely to increase the perception and emotional salience of tinnitus and loud sounds. This article is part of a Special Issue entitled: Tinnitus Neuroscience.
Copyright © 2012 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Andersson G, et al. Regional cerebral blood flow during tinnitus: a PET case study with lidocaine and auditory stimulation. Acta Otolaryngol. 2000;120:967–972. - PubMed
-
- Andersson G, et al. Tinnitus distress, anxiety, depression, and hearing problems among cochlear implant patients with tinnitus. Journal of the American Academy of Audiology. 2009;20:315–319. - PubMed
-
- Arnold W, et al. Focal metabolic activation in the predominant left auditory cortex in patients suffering from tinnitus: a PET study with [18F]deoxyglucose. ORL J Otorhinolaryngol Relat Spec. 1996;58:195–199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

