Malignant glioma: lessons from genomics, mouse models, and stem cells
- PMID: 22464322
- PMCID: PMC3719882
- DOI: 10.1016/j.cell.2012.03.009
Malignant glioma: lessons from genomics, mouse models, and stem cells
Abstract
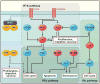
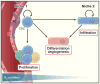
Eighty percent of malignant tumors that develop in the central nervous system are malignant gliomas, which are essentially incurable. Here, we discuss how recent sequencing studies are identifying unexpected drivers of gliomagenesis, including mutations in isocitrate dehydrogenase 1 and the NF-κB pathway, and how genome-wide analyses are reshaping the classification schemes for tumors and enhancing prognostic value of molecular markers. We discuss the controversies surrounding glioma stem cells and explore how the integration of new molecular data allows for the generation of more informative animal models to advance our knowledge of glioma's origin, progression, and treatment.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


References
-
- Anido J, Sáez-Borderías A, González-Juncà A, Rodón L, Folch G, Carmona MA, Prieto-Sánchez RM, Barba I, Martínez-Sáez E, Prudkin L, et al. TGF-β Receptor Inhibitors Target the CD44(high)/Id1(high) Glioma-Initiating Cell Population in Human Glioblastoma. Cancer Cell. 2010;18:655–668. - PubMed
-
- Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Tang Y, DeFrances J, Stover E, Weissleder R, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

