COSA-1 reveals robust homeostasis and separable licensing and reinforcement steps governing meiotic crossovers
- PMID: 22464324
- PMCID: PMC3339199
- DOI: 10.1016/j.cell.2012.01.052
COSA-1 reveals robust homeostasis and separable licensing and reinforcement steps governing meiotic crossovers
Abstract
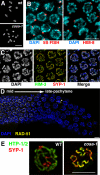
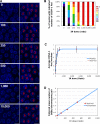
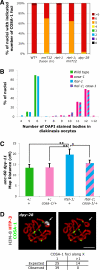
Crossovers (COs) between homologous chromosomes ensure their faithful segregation during meiosis. We identify C. elegans COSA-1, a cyclin-related protein conserved in metazoa, as a key component required to convert meiotic double-strand breaks (DSBs) into COs. During late meiotic prophase, COSA-1 localizes to foci that correspond to the single CO site on each homolog pair and indicate sites of eventual concentration of other conserved CO proteins. Chromosomes gain and lose competence to load CO proteins during meiotic progression, with competence to load COSA-1 requiring prior licensing. Our data further suggest a self-reinforcing mechanism maintaining CO designation. Modeling of a nonlinear dose-response relationship between IR-induced DSBs and COSA-1 foci reveals efficient conversion of DSBs into COs when DSBs are limiting and a robust capacity to limit cytologically differentiated CO sites when DSBs are in excess. COSA-1 foci serve as a unique live cell readout for investigating CO formation and CO interference.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




Comment in
-
The joy of six: how to control your crossovers.Cell. 2012 Mar 30;149(1):11-2. doi: 10.1016/j.cell.2012.03.011. Cell. 2012. PMID: 22464316
References
-
- Agarwal S, Roeder GS. Zip3 provides a link between recombination enzymes and synaptonemal complex proteins. Cell. 2000;102:245–255. - PubMed
-
- Baudat F, de Massy B. Regulating double-stranded DNA break repair towards crossover or non-crossover during mammalian meiosis. Chromosome Res. 2007;15:565–577. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

