Relative contribution of CTR1 and DMT1 in copper transport by the blood-CSF barrier: implication in manganese-induced neurotoxicity
- PMID: 22465424
- PMCID: PMC3336026
- DOI: 10.1016/j.taap.2012.03.006
Relative contribution of CTR1 and DMT1 in copper transport by the blood-CSF barrier: implication in manganese-induced neurotoxicity
Abstract
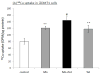
The homeostasis of copper (Cu) in the cerebrospinal fluid (CSF) is partially regulated by the Cu transporter-1 (CTR1) and divalent metal transporter-1 (DMT1) at the blood-CSF barrier (BCB) in the choroid plexus. Data from human and animal studies suggest an increased Cu concentration in blood, CSF, and brains following in vivo manganese (Mn) exposure. This study was designed to investigate the relative role of CTR1 and DMT1 in Cu transport under normal or Mn-exposed conditions using an immortalized choroidal Z310 cell line. Mn exposure in vitro resulted in an increased cellular 64Cu uptake and the up-regulation of both CTR1 and DMT1. Knocking down CTR1 by siRNA counteracted the Mn-induced increase of 64Cu uptake, while knocking down DMT1 siRNA resulted in an increased cellular 64Cu uptake in Mn-exposed cells. To distinguish the roles of CTR1 and DMT1 in Cu transport, the Z310 cell-based tetracycline (Tet)-inducible CTR1 and DMT1 expression cell lines were developed, namely iZCTR1 and iZDMT1 cells, respectively. In iZCTR1 cells, Tet induction led to a robust increase (25 fold) of 64Cu uptake with the time course corresponding to the increased CTR1. Induction of DMT1 by Tet in iZDMT1 cells, however, resulted in only a slight increase of 64Cu uptake in contrast to a substantial increase in DMT1 mRNA and protein expression. These data indicate that CTR1, but not DMT1, plays an essential role in transporting Cu by the BCB in the choroid plexus. Mn-induced cellular overload of Cu at the BCB is due, primarily, to Mn-induced over-expression of CTR1.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that there is no any conflict of interest with regards to financial, personal, or their relationships with other people or organizations for the studies presented in this article.
Figures





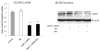
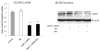









References
-
- Bertinato J, Swist E, Plouffe LJ, Brooks SP, L’abbé MR. Ctr2 is partially localized to the plasma membrane and stimulates copper uptake in COS-7 cells. Biochem J. 2008;409:731–740. - PubMed
-
- Boll MC, Alcaraz-Zubeldia M, Montes S, Rios C. Free copper, ferroxidase and SOD1 activities, lipid peroxidation and NO(x) content in the CSF. A different marker profile in four neurodegenerative diseases. Neurochem Res. 2008;33:1717–1723. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

