Differential state-dependent modification of inactivation-deficient Nav1.6 sodium channels by the pyrethroid insecticides S-bioallethrin, tefluthrin and deltamethrin
- PMID: 22465659
- PMCID: PMC3574822
- DOI: 10.1016/j.neuro.2012.03.007
Differential state-dependent modification of inactivation-deficient Nav1.6 sodium channels by the pyrethroid insecticides S-bioallethrin, tefluthrin and deltamethrin
Abstract
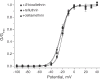
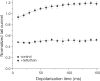
Pyrethroid insecticides disrupt nerve function by modifying the gating kinetics of transitions between the conducting and nonconducting states of voltage-gated sodium channels. Pyrethroids modify rat Na(v)1.6+β1+β2 channels expressed in Xenopus oocytes in both the resting state and in one or more states that require channel activation by repeated depolarization. The state dependence of modification depends on the pyrethroid examined: deltamethrin modification requires repeated channel activation, tefluthrin modification is significantly enhanced by repeated channel activation, and S-bioallethrin modification is unaffected by repeated activation. Use-dependent modification by deltamethrin and tefluthrin implies that these compounds bind preferentially to open channels. We constructed the rat Na(v)1.6Q3 cDNA, which contained the IFM/QQQ mutation in the inactivation gate domain that prevents fast inactivation and results in a persistently open channel. We expressed Na(v)1.6Q3+β1+β2 sodium channels in Xenopus oocytes and assessed the modification of open channels by pyrethroids by determining the effect of depolarizing pulse length on the normalized conductance of the pyrethroid-induced sodium tail current. Deltamethrin caused little modification of Na(v)1.6Q3 following short (10ms) depolarizations, but prolonged depolarizations (up to 150ms) caused a progressive increase in channel modification measured as an increase in the conductance of the pyrethroid-induced sodium tail current. Modification by tefluthrin was clearly detectable following short depolarizations and was increased by long depolarizations. By contrast modification by S-bioallethrin following short depolarizations was not altered by prolonged depolarization. These studies provide direct evidence for the preferential binding of deltamethrin and tefluthrin (but not S-bioallethrin) to Na(v)1.6Q3 channels in the open state and imply that the pyrethroid receptor of resting and open channels occupies different conformations that exhibit distinct structure-activity relationships.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflicts of interest with regard to sources of funding for this research or the design and interpretation of the experiments described herein.
Figures





References
-
- Bloomquist JR. Neuroreceptor mechanisms in pyrethroid mode of action and resistance. In: Roe M, Kuhr RJ, editors. Reviews in pesticide toxicology. Raleigh, NC: Toxicology Communications; 1993. pp. 181–226.
-
- Choi J-S, Soderlund DM. Structure–activity relationships for the action of 11 pyrethroid insecticides on rat Nav1.8 sodium channels expressed in Xenopus oocytes. Toxicol Appl Pharmacol. 2006;211:233–44. - PubMed
-
- de Weille JR, Vijverberg HPM, Narahashi T. Interactions of pyrethroids and octylguanidine with sodium channels of squid giant axons. Brain Res. 1988;445:1–11. - PubMed
-
- Dietrich PS, McGivern JG, Delgado SG, Koch BD, Eglen RM, Hunter JC, et al. Functional analysis of a voltage-gated sodium channel and its splice variant from rat dorsal root ganglia. J Neurochem. 1998;70:2262–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

