Chronic exposure to methylphenidate impairs appendicular bone quality in young rats
- PMID: 22465849
- PMCID: PMC3352964
- DOI: 10.1016/j.bone.2012.03.011
Chronic exposure to methylphenidate impairs appendicular bone quality in young rats
Abstract
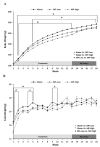
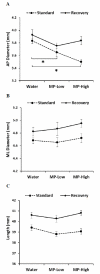
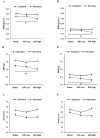
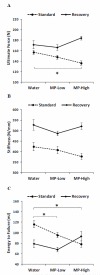
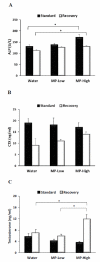
Methylphenidate (MP) is a psychostimulant widely prescribed to treat Attention Deficit Hyperactivity Disorder (ADHD). Although generally well tolerated, growth deficits have been reported in children and adolescents undergoing MP treatment. This study was designed to elucidate the skeletal effects of chronic MP administration in adolescent rats. Male, 4-week-old rats received one of two doses of MP (MP-Low or MP-High) delivered for 8 h a day via drinking water, or were untreated (water only). After 13 weeks, half were sacrificed (N=12/group) and the remaining rats were left to recover, untreated for 5 additional weeks. Femora, tibiae, and L5 vertebra were analyzed using calipers, DXA, and mechanical testing. Immediately following treatment, MP decreased femoral anterior-posterior diameter (5% and 9% for MP-Low and MP-High, respectively), femoral and tibial bone mineral density (BMD) (6% and 5% for MP-High femora and tibiae, respectively), and bone mineral content (BMC) (9% for MP-High femora and tibiae). In addition, femora from MP treated rats had reduced ultimate force (20% for MP-High) and energy to failure (20% and 33% for MP-Low and MP-High, respectively). However, after recovery, there were no statistically significant differences for any measured parameters. Despite these effects on the appendicular skeleton, no differences were identified between vertebral samples at either time-point. In summary, MP treatment resulted in smaller, less mineralized, and weaker bones at appendicular sites, but did not affect the axial site. Although these effects were ameliorated within 5 weeks, these data suggest that adolescents undergoing MP treatment may be at an increased risk for long bone fractures.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
References
-
- Dopheide JA, Pliszka SR. Attention Deficit Hyperactivity Disorder: An Update. Pharmacotherapy. 2009;29:656–679. - PubMed
-
- Loe IM, Feldman HM. Academic and educational outcomes of children with ADHD. J. Pediatr. Psychol. 2007;32:643–654. - PubMed
-
- Edwards G, Barkley RA, Laneri M, Fletcher K, Metevia L. Parent-adolescent conflict in teenagers with ADHD and ODD. J. Abnorm. Child. Psychol. 2001;29:557–572. - PubMed
-
- Konofal E, Lecendreux M, Cortese S. Sleep and ADHD. Sleep. Med. 2010;11:652–658. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

