Oscillatory dynamics in the hippocampus support dentate gyrus–CA3 coupling
- PMID: 22466505
- PMCID: PMC3378654
- DOI: 10.1038/nn.3081
Oscillatory dynamics in the hippocampus support dentate gyrus–CA3 coupling
Abstract
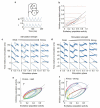
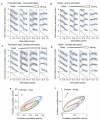
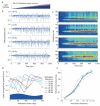
Gamma oscillations in the dentate gyrus and hippocampal CA3 show variable coherence in vivo, but the mechanisms and relevance for information flow are unknown. We found that carbachol-induced oscillations in rat CA3 have biphasic phase-response curves, consistent with the ability to couple with oscillations in afferent projections. Differences in response to stimulation of either the intrinsic feedback circuit or the dentate gyrus were well described by varying an impulse vector in a two-dimensional dynamical system, representing the relative input to excitatory and inhibitory neurons. Responses to sinusoidally modulated optogenetic stimulation confirmed that the CA3 network oscillation can entrain to periodic inputs, with a steep dependence of entrainment phase on input frequency. CA3 oscillations are therefore suited to coupling with oscillations in the dentate gyrus over a broad range of frequencies.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

