All-trans-retinoid acid induces the differentiation of encapsulated mouse embryonic stem cells into GABAergic neurons
- PMID: 22466603
- PMCID: PMC3334465
- DOI: 10.1016/j.diff.2012.03.001
All-trans-retinoid acid induces the differentiation of encapsulated mouse embryonic stem cells into GABAergic neurons
Abstract
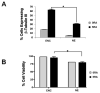
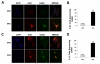
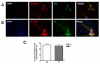
Embryonic stem (ES) cells are pluripotent cells that can differentiate into all three main germ layers: endoderm, mesoderm, and ectoderm. Although a number of methods have been developed to differentiate ES cells into neuronal phenotypes such as sensory and motor neurons, the efficient generation of GABAergic interneurons from ES cells still presents an ongoing challenge. Because the main output of inhibitory GABAergic interneurons is the gamma-aminobutyric-acid (GABA), a neurotransmitter whose controlled homeostasis is required for normal brain function, the efficient generation in culture of functional interneurons may have future implications on the treatment of neurological disorders such as epilepsy, autism, and schizophrenia. The goal of this work was to examine the generation of GABAergic neurons from mouse ES cells by comparing an embryoid body-based methodology versus a hydrogel-based encapsulation protocol that involves the use of all-trans-retinoid acid (RA). We observed that (1) there was a 2-fold increase in neuronal differentiation in encapsulated versus non-encapsulated cells and (2) there was an increase in the specificity for interneuronal differentiation in encapsulated cells, as assessed by mRNA expression and electrophysiology approaches. Furthermore, our results indicate that most of the neurons obtained from encapsulated mouse ES cells are GABA-positive (∼87%). Thus, these results suggest that combining encapsulation of ES cells and RA treatment provide a more efficient and scalable differentiation strategy for the generation in culture of functional GABAergic interneurons. This technology may have implications for future cell replacement therapies and the treatment of CNS disorders.
Published by Elsevier B.V.
Figures



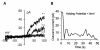
References
-
- Akasha AA, Sotiriadou I, Doss MX, Halbach M, Winkler J, Baunach JJ, Katsen-Globa A, Zimmermann H, Choo Y, Hescheler J, Sachinidis A. Entrapment of embryonic stem cells-derived cardiomyocytes in macroporous biodegradable microspheres: preparation and characterization. Cell. Physiol. Biochem. 2008;22:665–672. - PubMed
-
- Ali SA, Pappas IS, Parnavelas JG. Collagen type IV promotes the differentiation of neuronal progenitors and inhibits astroglial differentiation in cortical cell cultures. Dev. Brain Res. 1998;110:31–38. - PubMed
-
- Bailey AJ, Paul RG. Collagen: a not so simple protein. J Soc Leather Technol Chem. 1998;82:104–110.
-
- Bain G, Ray WJ, Yao M, Gottlieb DI. Retinoic acid promotes neural and represses mesodermal gene expression in mouse embryonic stem cells in culture. Biochem. Biophys. Res. Commun. 1996;223:691–694. - PubMed
-
- Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev. Biol. 1995;168:342–357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

