V-ATPase subunit ATP6AP1 (Ac45) regulates osteoclast differentiation, extracellular acidification, lysosomal trafficking, and protease exocytosis in osteoclast-mediated bone resorption
- PMID: 22467241
- PMCID: PMC3951719
- DOI: 10.1002/jbmr.1623
V-ATPase subunit ATP6AP1 (Ac45) regulates osteoclast differentiation, extracellular acidification, lysosomal trafficking, and protease exocytosis in osteoclast-mediated bone resorption
Abstract
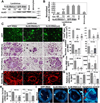
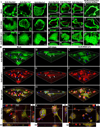
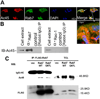
Lysosomal trafficking and protease exocytosis in osteoclasts are essential for ruffled border formation and bone resorption. Yet the mechanism underlying lysosomal trafficking and the related process of exocytosis remains largely unknown. We found ATP6ap1 (Ac45), an accessory subunit of vacuolar-type H(+)-ATPases (V-ATPases), to be highly induced by receptor activator for nuclear factor kappa B ligand (RANKL) in osteoclast differentiation. Ac45 knockdown osteoclasts formed normal actin rings, but had severely impaired extracellular acidification and bone resorption. Ac45 knockdown significantly reduced osteoclast formation. The decrease in the number of osteoclasts does not result from abnormal apoptosis; rather, it results from decreased osteoclast precursor cell proliferation and fusion, which may be partially due to the downregulation of extracellular signal-regulated kinase (ERK) phosphorylation and FBJ osteosarcoma oncogene (c-fos), nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1), and "transmembrane 7 superfamily member 4" (Tm7sf4) expression. Notably, Ac45 knockdown osteoclasts exhibited impaired lysosomal trafficking and exocytosis, as indicated by the absence of lysosomal trafficking to the ruffled border and a lack of cathepsin K exocytosis into the resorption lacuna. Our data revealed that the impaired exocytosis is specifically due to Ac45 deficiency, and not the general consequence of a defective V-ATPase. Together, our results demonstrate the essential role of Ac45 in osteoclast-mediated extracellular acidification and protease exocytosis, as well as the ability of Ac45 to guide lysosomal intracellular trafficking to the ruffled border, potentially through its interaction with the small guanosine-5'-triphosphatase (GTPase) Rab7. Our work indicates that Ac45 may be a novel therapeutic target for osteolytic disease.
Copyright © 2012 American Society for Bone and Mineral Research.
Conflict of interest statement
All authors state that they have no conflicts of interest.
Figures




Similar articles
-
Silencing of Ac45 Simultaneously Inhibits Osteoclast-Mediated Bone Resorption and Attenuates Dendritic Cell-Mediated Inflammation through Impairing Acidification and Cathepsin K Secretion.Infect Immun. 2020 Dec 15;89(1):e00436-20. doi: 10.1128/IAI.00436-20. Print 2020 Dec 15. Infect Immun. 2020. PMID: 33077625 Free PMC article.
-
Cytoplasmic terminus of vacuolar type proton pump accessory subunit Ac45 is required for proper interaction with V(0) domain subunits and efficient osteoclastic bone resorption.J Biol Chem. 2008 May 9;283(19):13194-204. doi: 10.1074/jbc.M709712200. Epub 2008 Jan 28. J Biol Chem. 2008. PMID: 18227071 Free PMC article.
-
Atp6v0d2 is an essential component of the osteoclast-specific proton pump that mediates extracellular acidification in bone resorption.J Bone Miner Res. 2009 May;24(5):871-85. doi: 10.1359/jbmr.081239. J Bone Miner Res. 2009. PMID: 19113919 Free PMC article.
-
V-ATPase a3 Subunit in Secretory Lysosome Trafficking in Osteoclasts.Biol Pharm Bull. 2022;45(10):1426-1431. doi: 10.1248/bpb.b22-00371. Biol Pharm Bull. 2022. PMID: 36184499 Review.
-
Structure and function of V-ATPases in osteoclasts: potential therapeutic targets for the treatment of osteolysis.Histol Histopathol. 2007 Apr;22(4):443-54. doi: 10.14670/HH-22.443. Histol Histopathol. 2007. PMID: 17290355 Review.
Cited by
-
TRAFD1 (FLN29) Interacts with Plekhm1 and Regulates Osteoclast Acidification and Resorption.PLoS One. 2015 May 19;10(5):e0127537. doi: 10.1371/journal.pone.0127537. eCollection 2015. PLoS One. 2015. PMID: 25992615 Free PMC article.
-
PPARα in lysosomal biogenesis: A perspective.Pharmacol Res. 2016 Jan;103:144-8. doi: 10.1016/j.phrs.2015.11.011. Epub 2015 Nov 24. Pharmacol Res. 2016. PMID: 26621249 Free PMC article.
-
C/ebpα controls osteoclast terminal differentiation, activation, function, and postnatal bone homeostasis through direct regulation of Nfatc1.J Pathol. 2018 Mar;244(3):271-282. doi: 10.1002/path.5001. Epub 2018 Jan 29. J Pathol. 2018. PMID: 29083488 Free PMC article.
-
C/EBPα transcription factor is regulated by the RANK cytoplasmic 535IVVY538 motif and stimulates osteoclastogenesis more strongly than c-Fos.J Biol Chem. 2018 Jan 26;293(4):1480-1492. doi: 10.1074/jbc.M116.736009. Epub 2017 Nov 9. J Biol Chem. 2018. PMID: 29122885 Free PMC article.
-
ATP6AP1 promotes cell proliferation and tamoxifen resistance in luminal breast cancer by inducing autophagy.Cell Death Dis. 2025 Mar 25;16(1):201. doi: 10.1038/s41419-025-07534-y. Cell Death Dis. 2025. PMID: 40133274 Free PMC article.
References
-
- Rousselle AV, Heymann D. Osteoclastic acidification pathways during bone resorption. Bone. 2002 Apr;30(4):533–540. - PubMed
-
- Akisaka T, Yoshida H, Suzuki R, Takama K. Adhesion structures and their cytoskeleton-membrane interactions at podosomes of osteoclasts in culture. Cell Tissue Res. 2008 Mar;331(3):625–641. - PubMed
-
- Palokangas H, Mulari M, Vaananen HK. Endocytic pathway from the basal plasma membrane to the ruffled border membrane in bone-resorbing osteoclasts. J.Cell Sci. 1997 Aug;110(Pt 15):1767–1780. - PubMed
-
- Mulari MT, Zhao H, Lakkakorpi PT, Vaananen HK. Osteoclast ruffled border has distinct subdomains for secretion and degraded matrix uptake. Traffic. 2003 Feb;4(2):113–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

