The PhoP/PhoQ system and its role in Serratia marcescens pathogenesis
- PMID: 22467788
- PMCID: PMC3370626
- DOI: 10.1128/JB.06820-11
The PhoP/PhoQ system and its role in Serratia marcescens pathogenesis
Abstract
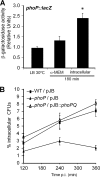
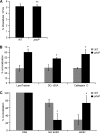
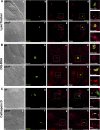
Serratia marcescens is able to invade, persist, and multiply inside nonphagocytic cells, residing in nonacidic, nondegradative, autophagosome-like vacuoles. In this work, we have examined the physiological role of the PhoP/PhoQ system and its function in the control of critical virulence phenotypes in S. marcescens. We have demonstrated the involvement of the PhoP/PhoQ system in the adaptation of this bacterium to growth on scarce environmental Mg(2+), at acidic pH, and in the presence of polymyxin B. We have also shown that these environmental conditions constitute signals that activate the PhoP/PhoQ system. We have found that the two S. marcescens mgtE orthologs present a conserved PhoP-binding motif and demonstrated that mgtE1 expression is PhoP dependent, reinforcing the importance of PhoP control in magnesium homeostasis. Finally, we have demonstrated that phoP expression is activated intracellularly and that a phoP mutant strain is defective in survival inside epithelial cells. We have shown that the Serratia PhoP/PhoQ system is involved in prevention of the delivery to degradative/acidic compartments.
Figures



References
-
- Alexeyev MF. 1999. The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of gram-negative bacteria. Biotechniques 26:824–826, 828 - PubMed
-
- Bailey TL, Elkan C. 1995. The value of prior knowledge in discovering motifs with MEME. Proc. Int. Conf. Intell. Syst. Mol. Biol. 3:21–29 - PubMed
-
- Bailey TL, Gribskov M. 1998. Methods and statistics for combining motif match scores. J. Comput. Biol. 5:211–221 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

