The F-BAR domains from srGAP1, srGAP2 and srGAP3 regulate membrane deformation differently
- PMID: 22467852
- PMCID: PMC3516379
- DOI: 10.1242/jcs.098962
The F-BAR domains from srGAP1, srGAP2 and srGAP3 regulate membrane deformation differently
Abstract
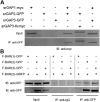
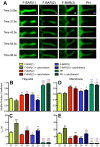
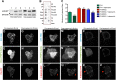
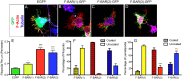
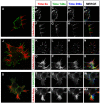
Coordination of membrane deformation and cytoskeletal dynamics lies at the heart of many biological processes critical for cell polarity, motility and morphogenesis. We have recently shown that Slit-Robo GTPase-activating protein 2 (srGAP2) regulates neuronal morphogenesis through the ability of its F-BAR domain to regulate membrane deformation and induce filopodia formation. Here, we demonstrate that the F-BAR domains of two closely related family members, srGAP1 and srGAP3 [designated F-BAR(1) and F-BAR(3), respectively] display significantly different membrane deformation properties in non-neuronal COS7 cells and in cortical neurons. F-BAR(3) induces filopodia in both cell types, though less potently than F-BAR(2), whereas F-BAR(1) prevents filopodia formation in cortical neurons and reduces plasma membrane dynamics. These three F-BAR domains can heterodimerize, and they act synergistically towards filopodia induction in COS7 cells. As measured by fluorescence recovery after photobleaching, F-BAR(2) displays faster molecular dynamics than F-BAR(3) and F-BAR(1) at the plasma membrane, which correlates well with its increased potency to induce filopodia. We also show that the molecular dynamic properties of F-BAR(2) at the membrane are partially dependent on F-Actin. Interestingly, acute phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P(2)] depletion in cells does not interfere with plasma membrane localization of F-BAR(2), which is compatible with our result showing that F-BAR(2) binds to a broad range of negatively-charged phospholipids present at the plasma membrane, including phosphatidylserine (PtdSer). Overall, our results provide novel insights into the functional diversity of the membrane deformation properties of this subclass of F-BAR-domains required for cell morphogenesis.
Figures


References
-
- Carlson B. R., Lloyd K. E., Kruszewski A., Kim I. H., Rodriguiz R. M., Heindel C., Faytell M., Dudek S. M., Wetsel W. C., Soderling S. H. (2011). WRP/srGAP3 facilitates the initiation of spine development by an inverse F-BAR domain, and its loss impairs long-term memory. J. Neurosci. 31, 2447–2460 10.1523/JNEUROSCI.4433-10.2011 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

