Polycomb repressive complex 2 impedes intestinal cell terminal differentiation
- PMID: 22467857
- PMCID: PMC3516381
- DOI: 10.1242/jcs.102061
Polycomb repressive complex 2 impedes intestinal cell terminal differentiation
Abstract
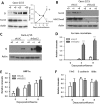
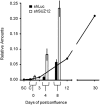
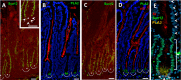
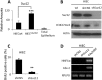
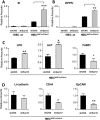
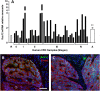
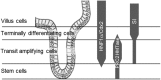
The crypt-villus axis constitutes the functional unit of the small intestine, where mature absorptive cells are confined to the villi, and stem cells and transit amplifying and differentiating cells are restricted to the crypts. The polycomb group (PcG) proteins repress differentiation and promote self-renewal in embryonic stem cells. PcGs prevent transcriptional activity by catalysing epigenetic modifications, such as the covalent addition of methyl groups on histone tails, through the action of the polycomb repressive complex 2 (PRC2). Although a role for PcGs in the preservation of stemness characteristics is now well established, recent evidence suggests that they may also be involved in the regulation of differentiation. Using intestinal epithelial cell models that recapitulate the enterocytic differentiation programme, we generated a RNAi-mediated stable knockdown of SUZ12, which constitutes a cornerstone for PRC2 assembly and functionality, in order to analyse intestinal cell proliferation and differentiation. Expression of SUZ12 was also investigated in human intestinal tissues, revealing the presence of SUZ12 in most proliferative epithelial cells of the crypt and an increase in its expression in colorectal cancers. Moreover, PRC2 disruption led to a significant precocious expression of a number of terminal differentiation markers in intestinal cell models. Taken together, our data identified a mechanism whereby PcG proteins participate in the repression of the enterocytic differentiation program, and suggest that a similar mechanism exists in situ to slow down terminal differentiation in the transit amplifying cell population.
Figures
References
-
- Barker N., Clevers H. (2010). Lineage tracing in the intestinal epithelium. In Current Protocols in Stem Cell Biology Ch. 5, Unit5A.4 - PubMed
-
- Basora N., Herring–Gillam F. E., Boudreau F., Perreault N., Pageot L. P., Simoneau M., Bouatrouss Y., Beaulieu J. F. (1999). Expression of functionally distinct variants of the beta(4)A integrin subunit in relation to the differentiation state in human intestinal cells. J. Biol. Chem. 274, 29819–29825 10.1074/jbc.274.42.29819 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

