Arsenic promotes ubiquitinylation and lysosomal degradation of cystic fibrosis transmembrane conductance regulator (CFTR) chloride channels in human airway epithelial cells
- PMID: 22467879
- PMCID: PMC3366821
- DOI: 10.1074/jbc.M111.338855
Arsenic promotes ubiquitinylation and lysosomal degradation of cystic fibrosis transmembrane conductance regulator (CFTR) chloride channels in human airway epithelial cells
Abstract
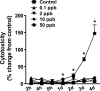
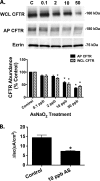
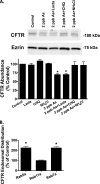
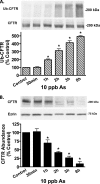
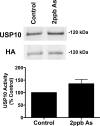
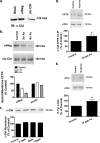
Arsenic exposure significantly increases respiratory bacterial infections and reduces the ability of the innate immune system to eliminate bacterial infections. Recently, we observed in the gill of killifish, an environmental model organism, that arsenic exposure induced the ubiquitinylation and degradation of cystic fibrosis transmembrane conductance regulator (CFTR), a chloride channel that is essential for the mucociliary clearance of respiratory pathogens in humans. Accordingly, in this study, we tested the hypothesis that low dose arsenic exposure reduces the abundance and function of CFTR in human airway epithelial cells. Arsenic induced a time- and dose-dependent increase in multiubiquitinylated CFTR, which led to its lysosomal degradation, and a decrease in CFTR-mediated chloride secretion. Although arsenic had no effect on the abundance or activity of USP10, a deubiquitinylating enzyme, siRNA-mediated knockdown of c-Cbl, an E3 ubiquitin ligase, abolished the arsenic-stimulated degradation of CFTR. Arsenic enhanced the degradation of CFTR by increasing phosphorylated c-Cbl, which increased its interaction with CFTR, and subsequent ubiquitinylation of CFTR. Because epidemiological studies have shown that arsenic increases the incidence of respiratory infections, this study suggests that one potential mechanism of this effect involves arsenic-induced ubiquitinylation and degradation of CFTR, which decreases chloride secretion and airway surface liquid volume, effects that would be proposed to reduce mucociliary clearance of respiratory pathogens.
Figures
References
-
- United States Department of Health and Human Services, Agency for Toxic Substances and Disease Registry (2011) ATSDR 2011 Priority List of Hazardous Substances, Atlanta GA
-
- Tseng C. H., Chong C. K., Chen C. J., Tai T. Y. (1996) Dose-response relationship between peripheral vascular disease and ingested inorganic arsenic among residents in blackfoot disease endemic villages in Taiwan. Atherosclerosis 120, 125–133 - PubMed
-
- Bates M. N., Rey O. A., Biggs M. L., Hopenhayn C., Moore L. E., Kalman D., Steinmaus C., Smith A. H. (2004) Case-control study of bladder cancer and exposure to arsenic in Argentina. Am. J. Epidemiol. 159, 381–389 - PubMed
-
- Ferreccio C., González C., Milosavjlevic V., Marshall G., Sancha A. M., Smith A. H. (2000) Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology 11, 673–679 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

