Intrathecal clonidine in the neonatal rat: dose-dependent analgesia and evaluation of spinal apoptosis and toxicity
- PMID: 22467896
- PMCID: PMC4455964
- DOI: 10.1213/ANE.0b013e3182501a09
Intrathecal clonidine in the neonatal rat: dose-dependent analgesia and evaluation of spinal apoptosis and toxicity
Abstract
Background: Neuraxial clonidine is used for perioperative analgesia in children of all ages. Preclinical studies in the postnatal rat allow comparison of the relative toxicity and safety of spinal analgesics throughout postnatal development.
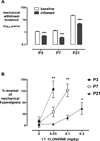
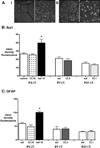
Methods: Rat pups aged 3, 7, or 21 postnatal (P) days were briefly anesthetized for intrathecal injections of saline or clonidine. At each age, the maximum tolerated, antinociceptive (increased hindlimb mechanical withdrawal threshold) and antihyperalgesic (hindpaw carrageenan inflammation) doses were determined. Lumbar spinal cord sections were assessed for apoptosis and cell death (histology, activated caspase-3 immunohistochemistry, Fluoro-Jade C staining), histopathology (hematoxylin and eosin staining), and increased glial reactivity (microglial and astrocytic markers). P3 intrathecal ketamine sections served as positive controls. In additional groups, thermal latency and mechanical withdrawal threshold were measured at P35.
Results: Intrathecal clonidine produces age- and dose-dependent analgesia in rat pups. Maximal doses of clonidine did not alter the degree or distribution of apoptosis or increase glial reactivity in the neonatal spinal cord. No spinal histopathology was seen 1 or 7 days after injection at any age. Intrathecal clonidine did not produce persistent changes in reflex sensitivity to mechanical or thermal stimuli at P35.
Conclusions: Intrathecal clonidine in the postnatal rat did not produce signs of spinal cord toxicity, even at doses much larger than required for analgesia. The therapeutic ratio (maximum tolerated dose/antihyperalgesic dose) was >300 at P3, >30 at P7, and >10 at P21. These data provide additional information to inform the clinical choice of spinal analgesic drug in early life.
Conflict of interest statement
Figures




References
-
- Reddy SV, Maderdrut JL, Yaksh TL. Spinal cord pharmacology of adrenergic agonist-mediated antinociception. J Pharmacol Exp Ther. 1980;213:525–533. - PubMed
-
- Yaksh TL, Reddy SV. Studies in the primate on the analgetic effects associated with intrathecal actions of opiates, alpha-adrenergic agonists and baclofen. Anesthesiology. 1981;54:451–467. - PubMed
-
- Yaksh TL. Pharmacology of spinal adrenergic systems which modulate spinal nociceptive processing. Pharmacol Biochem Behav. 1985;22:845–858. - PubMed
-
- Eisenach JC, De Kock M, Klimscha W. alpha(2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984–1995) Anesthesiology. 1996;85:655–674. - PubMed
-
- Eisenach JC, Lysak SZ, Viscomi CM. Epidural clonidine analgesia following surgery: phase I. Anesthesiology. 1989;71:640–646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

