Joint modelling of longitudinal outcome and interval-censored competing risk dropout in a schizophrenia clinical trial
- PMID: 22468033
- PMCID: PMC3315284
- DOI: 10.1111/j.1467-985X.2011.00719.x
Joint modelling of longitudinal outcome and interval-censored competing risk dropout in a schizophrenia clinical trial
Abstract
The 'Clinical antipsychotic trials in intervention effectiveness' study, was designed to evaluate whether there were significant differences between several antipsychotic medications in effectiveness, tolerability, cost and quality of life of subjects with schizophrenia. Overall, 74 % of patients discontinued the study medication for various reasons before the end of 18 months in phase I of the study. When such a large percentage of study participants fail to complete the study schedule, it is not clear whether the apparent profile in effectiveness reflects genuine changes over time or is influenced by selection bias, with participants with worse (or better) outcome values being more likely to drop out or to discontinue. To assess the effect of dropouts for different reasons on inferences, we construct a joint model for the longitudinal outcome and cause-specific dropouts that allows for interval-censored dropout times. Incorporating the information regarding the cause of dropout improves inferences and provides better understanding of the association between cause-specific dropout and the outcome process. We use simulations to demonstrate the advantages of the joint modelling approach in terms of bias and efficiency.
Figures
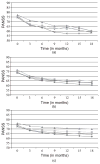
 olanzapine;
olanzapine;
 quetiapine;
quetiapine;
 , risperidone;
, risperidone;
 , perphenazine
, perphenazine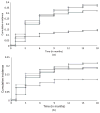

 olanzapine; quetiapine;
olanzapine; quetiapine;
 , risperidone;
, risperidone;
 , perphenazine
, perphenazine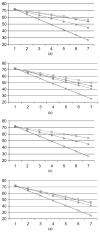
 , truth;
, truth;
 , joint model, separate dropout;
, joint model, separate dropout;
 , joint model, common dropout;
, joint model, common dropout;
 , separate model
, separate modelReferences
-
- Brown ER, Ibrahim JG. A Bayesian semiparametric joint hierarchical model for longitudinal and survival data. Biometrics. 2003;59:221–228. - PubMed
-
- Diggle P, Farewell D, Henderson R. Analysis of longitudinal data with drop-out: objectives, assumptions and a proposal (with discussion) Appl Statist. 2007;56:499–550.
-
- Diggle P, Sousa I, Chetwynd A. Joint modelling of repeated measurements and time-to-event outcomes: the Fourth Armitage Lecture. Statist Med. 2008;27:2981–2998. - PubMed
-
- Dobson A, Henderson R. Diagnostics for joint longitudinal and dropout time modelling. Biometrics. 2003;59:741–751. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
