Impact of Baseline Disease Severity Over 26 and 52 Weeks of Treatment with Calcitriol Ointment 3µg/g in Patients with Mild-to-moderate Plaque Psoriasis
- PMID: 22468170
- PMCID: PMC3315885
Impact of Baseline Disease Severity Over 26 and 52 Weeks of Treatment with Calcitriol Ointment 3µg/g in Patients with Mild-to-moderate Plaque Psoriasis
Abstract
Objective: Calcitriol 3µg/g ointment has been shown to be a safe and effective treatment for adults with mild-to-moderate plaque psoriasis. This analysis evaluated the response to calcitriol 3µg/g ointment relative to baseline disease.
Design: Retrospective analysis of data from a 12-month safety and tolerability trial.
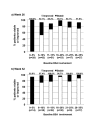
Setting and participants: At baseline, 40.1 percent (130/324) of patients had an affected body surface area of 11 to 20 percent, and 55.2 percent (179/324) had moderate and 25.9 percent (84/324) had severe disease according to global severity score. Patients applied calcitriol 3µg/g ointment twice daily for up to 52 weeks.
Measurements: Change in investigator's global severity scores and involved body surface area at Week 26 (N=249) and Week 52 (N=130) relative to baseline.
Results: Compared with baseline, most patients experienced at least a 1-grade improvement in global severity score at Weeks 26 (195/249, 78.3%) and 52 (109/130, 83.8%). Stabilization (i.e., no change in global severity score) was reported in 19.3 percent (48/249) at Week 26 and in 12.3 percent (16/130) at Week 52. Most patients also experienced at least a 1-grade improvement in body surface area involved at Weeks 26 (152/249, 61.0%) and 52 (95/130, 73.1%). Stabilization (no change in affected body surface area) was reported in 32.5 percent (81/249) at Week 26 and 24.6 percent (32/130) at Week 52. The proportion of patients experiencing improvement in global severity score and body surface area was comparable across all categories of severity and disease extent at baseline.
Conclusion: This analysis suggests that calcitriol 3µg/g ointment use for 26 weeks (N=249) and 52 weeks (N=130) was associated with disease improvement or stabilization in most patients with plaque psoriasis.
Conflict of interest statement
Figures

Similar articles
-
Calcitriol 3 microg/g ointment in the management of mild to moderate plaque type psoriasis: results from 2 placebo-controlled, multicenter, randomized double-blind, clinical studies.J Drugs Dermatol. 2007 Apr;6(4):428-35. J Drugs Dermatol. 2007. PMID: 17668541 Clinical Trial.
-
Calcitriol ointment and clobetasol propionate cream: a new regimen for the treatment of plaque psoriasis.Eur J Dermatol. 2003 May-Jun;13(3):261-5. Eur J Dermatol. 2003. PMID: 12804986 Clinical Trial.
-
Calcitriol ointment 3 microg/g is safe and effective over 52 weeks for the treatment of mild to moderate plaque psoriasis.Cutis. 2009 Apr;83(4):205-12. Cutis. 2009. PMID: 19445311 Clinical Trial.
-
Calcitriol 3 microg/g ointment: an effective and safe addition to the armamentarium in topical psoriasis therapy.J Drugs Dermatol. 2009 Aug;8(8 Suppl):s17-22. J Drugs Dermatol. 2009. PMID: 19702032 Review.
-
Efficacy and safety of topical calcitriol 3 microg/g ointment, a new topical therapy for chronic plaque psoriasis.J Drugs Dermatol. 2009 Aug;8(8 Suppl):s9-16. J Drugs Dermatol. 2009. PMID: 19702031 Review.
Cited by
-
Oral and Topical Vitamin D, Sunshine, and UVB Phototherapy Safely Control Psoriasis in Patients with Normal Pretreatment Serum 25-Hydroxyvitamin D Concentrations: A Literature Review and Discussion of Health Implications.Nutrients. 2021 Apr 29;13(5):1511. doi: 10.3390/nu13051511. Nutrients. 2021. PMID: 33947070 Free PMC article. Review.
References
-
- Koo J. Population-based epidemiologic study of psoriasis with emphasis on quality of life assessment. Dermatol Clin. 1996;14(3):485–496. - PubMed
-
- Rapp SR, Feldman SR, Exum ML, Fleischer AB, Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3 Pt 1):401–407. - PubMed
-
- Rapp SR, Cottrell CA, Leary MR. Social coping strategies associated with quality of life decrements among psoriasis patients. Br J Dermatol. 2001;145(4):610–616. - PubMed
-
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60(4):643–659. - PubMed
LinkOut - more resources
Full Text Sources
