An overview of the development of combined oral contraceptives containing estradiol: focus on estradiol valerate/dienogest
- PMID: 22468839
- PMCID: PMC3399636
- DOI: 10.3109/09513590.2012.662547
An overview of the development of combined oral contraceptives containing estradiol: focus on estradiol valerate/dienogest
Abstract
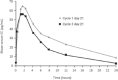
Natural estrogens such as estradiol (E(2)) or its valerate ester (E(2)V) offer an alternative to ethinyl estradiol (EE). E(2)-containing combined oral contraceptives (COCs) have demonstrated sufficient ovulation inhibition and acceptable contraceptive efficacy. However, earlier formulations were generally associated with unacceptable bleeding profiles. Two E(2)V-containing preparations have been approved to date for contraceptive use: E(2)V/cyproterone acetate (CPA) (Femilar(®); only approved in Finland and only in women >40 years or women aged 35-40 years in whom a COC containing EE is not appropriate) and E(2)V/dienogest (DNG; Qlaira(®)/Natazia(®)). The objective of the current review is to provide an overview of the development of COCs containing natural estrogen, highlighting past issues and challenges faced by earlier formulations, as well as the current status and future directions. The majority of information to date pertains to the development of E(2)V/DNG.
Figures



References
-
- Lobo RA, Stanczyk FZ. New knowledge in the physiology of hormonal contraceptives. Am J Obstet Gynecol. 1994;170:1499–1507. - PubMed
-
- Düsterberg B, Ellman H, Müller U, Rowe E, Mühe B. A three-year clinical investigation into efficacy, cycle control and tolerability of a new low-dose monophasic oral contraceptive containing gestodene. Gynecol Endocrinol. 1996;10:33–39. - PubMed
-
- Huber J, Foidart JM, Wuttke W, Merki-Feld GS, The HS, Gerlinger C, Schellschmidt I, Heithecker R. Efficacy and tolerability of a monophasic oral contraceptive containing ethinylestradiol and drospirenone. Eur J Contracept Reprod Health Care. 2000;5:25–34. - PubMed
-
- Sitruk-Ware R. New progestagens for contraceptive use. Hum Reprod Update. 2006;12:169–178. - PubMed
-
- Spona J, Feichtinger W, Kindermann C, Moore C, Mellinger U, Walter F, Gräser T. Modulation of ovarian function by an oral contraceptive containing 30 micrograms ethinyl estradiol in combination with 2.00 mgdienogest. Contraception. 1997;56:185–191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
