Human skeletal muscle fiber type specific protein content
- PMID: 22469996
- PMCID: PMC3358799
- DOI: 10.1016/j.ab.2012.03.018
Human skeletal muscle fiber type specific protein content
Abstract
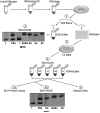
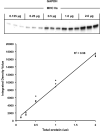
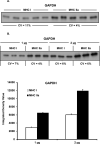
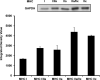
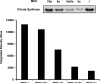
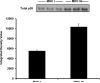
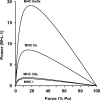
The aim of this project was to develop a method to assess fiber type specific protein content across the continuum of human skeletal muscle fibers. Individual vastus lateralis muscle fibers (n = 264) were clipped into two portions: one for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) fiber typing and one for Western blot protein identification. Following fiber type determination, fiber segments were combined into fiber type specific pools (∼20 fibers/pool) and measured for total protein quantity, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), citrate synthase (CS), and total p38 content. GAPDH content was 64, 54, 160, and 138% more abundant in myosin heavy chain (MHC) I/IIa, MHC IIa, MHC IIa/IIx, and MHC IIx fibers, respectively, when compared with MHC I. Inversely, CS content was 528, 472, 242, and 47% more abundant in MHC I, MHC I/IIa, MHC IIa, and MHC IIa/IIx fibers, respectively, when compared with MHC IIx. Total p38 content was 87% greater in MHC IIa versus MHC I fibers. These data and this approach establish a reliable method for human skeletal muscle fiber type specific protein analysis. Initial results show that particular proteins exist in a hierarchal fashion throughout the continuum of human skeletal muscle fiber types, further highlighting the necessity of fiber type specific analysis.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
References
-
- Saltin B, Gollnick P. Handbook of Physiology. Skeletal Muscle. Am. Physiol. Soc.; Bethesda, MD: 1983. Skeletal muscle adaptability: significance for metabolism and performance.
-
- Bottinelli R, Pellegrino MA, Canepari M, Rossi R, Reggiani C. Specific contributions of various muscle fibre types to human muscle performance: an in vitro study. J Electromyogr Kinesiol. 1999;9:87–95. - PubMed
-
- Smerdu V, Karsch-Mizrachi I, Campione M, Leinwand L, Schiaffino S. Type IIx myosin heavy chain transcripts are expressed in type IIb fibers of human skeletal muscle. Am J Physiol. 1994;267:C1723–8. - PubMed
-
- Billeter R, Heizmann CW, Howald H, Jenny E. Analysis of myosin light and heavy chain types in single human skeletal muscle fibers. Eur J Biochem. 1981;116:389–95. - PubMed
-
- Gollnick PD, Armstrong RB, Saltin B, Saubert C.W.t., Sembrowich WL, Shepherd RE. Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol. 1973;34:107–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

