Pharmacokinetic/Pharmacodynamic predictors of clinical potency for hepatitis C virus nonnucleoside polymerase and protease inhibitors
- PMID: 22470110
- PMCID: PMC3370793
- DOI: 10.1128/AAC.06283-11
Pharmacokinetic/Pharmacodynamic predictors of clinical potency for hepatitis C virus nonnucleoside polymerase and protease inhibitors
Abstract
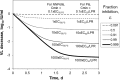
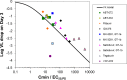
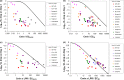
This analysis was conducted to determine whether the hepatitis C virus (HCV) viral kinetics (VK) model can predict viral load (VL) decreases for nonnucleoside polymerase inhibitors (NNPolIs) and protease inhibitors (PIs) after 3-day monotherapy studies of patients infected with genotype 1 chronic HCV. This analysis includes data for 8 NNPolIs and 14 PIs, including VL decreases from 3-day monotherapy, total plasma trough concentrations on day 3 (C(min)), replicon data (50% effective concentration [EC(50)] and protein-shifted EC(50) [EC(50,PS)]), and for PIs, liver-to-plasma ratios (LPRs) measured in vivo in preclinical species. VK model simulations suggested that achieving additional log(10) VL decreases greater than one required 10-fold increases in the C(min). NNPolI and PI data further supported this result. The VK model was successfully used to predict VL decreases in 3-day monotherapy for NNPolIs based on the EC(50,PS) and the day 3 C(min). For PIs, however, predicting VL decreases using the same model and the EC(50,PS) and day 3 C(min) was not successful; a model including LPR values and the EC(50) instead of the EC(50,PS) provided a better prediction of VL decrease. These results are useful for designing phase 1 monotherapy studies for NNPolIs and PIs by clarifying factors driving VL decreases, such as the day 3 C(min) and the EC(50,PS) for NNPolIs or the EC(50) and LPR for PIs. This work provides a framework for understanding the pharmacokinetic/pharmacodynamic relationship for other HCV drug classes. The availability of mechanistic data on processes driving the target concentration, such as liver uptake transporters, should help to improve the predictive power of the approach.
Figures
Similar articles
-
Estimation of inhibitory quotient using a comparative equilibrium dialysis assay for prediction of viral response to hepatitis C virus inhibitors.J Viral Hepat. 2011 May;18(5):338-48. doi: 10.1111/j.1365-2893.2010.01314.x. J Viral Hepat. 2011. PMID: 20456634
-
Characterization of vaniprevir, a hepatitis C virus NS3/4A protease inhibitor, in patients with HCV genotype 1 infection: safety, antiviral activity, resistance, and pharmacokinetics.Antiviral Res. 2013 Sep;99(3):214-20. doi: 10.1016/j.antiviral.2013.05.015. Epub 2013 Jun 7. Antiviral Res. 2013. PMID: 23747481 Clinical Trial.
-
Antiviral activity of boceprevir monotherapy in treatment-naive subjects with chronic hepatitis C genotype 2/3.J Hepatol. 2013 Jul;59(1):31-7. doi: 10.1016/j.jhep.2013.02.018. Epub 2013 Feb 27. J Hepatol. 2013. PMID: 23454058 Clinical Trial.
-
Antiviral resistance and the future landscape of hepatitis C virus infection therapy.J Infect Dis. 2013 Mar;207 Suppl 1:S33-9. doi: 10.1093/infdis/jis761. J Infect Dis. 2013. PMID: 23390303 Review.
-
Recent advances in hepatitis C virus treatment: review of HCV protease inhibitor clinical trials.Rev Recent Clin Trials. 2010 Sep;5(3):158-73. doi: 10.2174/157488710792007293. Rev Recent Clin Trials. 2010. PMID: 20482493 Review.
Cited by
-
Discovery of N-[4-[6-tert-butyl-5-methoxy-8-(6-methoxy-2-oxo-1H-pyridin-3-yl)-3-quinolyl]phenyl]methanesulfonamide (RG7109), a potent inhibitor of the hepatitis C virus NS5B polymerase.J Med Chem. 2014 Mar 13;57(5):1914-31. doi: 10.1021/jm401329s. Epub 2013 Nov 6. J Med Chem. 2014. PMID: 24195700 Free PMC article.
-
HCV Kinetic Models and Their Implications in Drug Development.CPT Pharmacometrics Syst Pharmacol. 2015 Apr;4(4):231-42. doi: 10.1002/psp4.28. Epub 2015 Apr 17. CPT Pharmacometrics Syst Pharmacol. 2015. PMID: 26225247 Free PMC article. Review.
-
A Comprehensive Review of the Clinical Pharmacokinetics, Pharmacodynamics, and Drug Interactions of Nirmatrelvir/Ritonavir.Clin Pharmacokinet. 2024 Jan;63(1):27-42. doi: 10.1007/s40262-023-01339-y. Epub 2024 Jan 4. Clin Pharmacokinet. 2024. PMID: 38177893 Free PMC article. Review.
-
Characterization of HIV-1 entry inhibitors with broad activity against R5 and X4 viral strains.J Transl Med. 2015 Apr 2;13:107. doi: 10.1186/s12967-015-0461-9. J Transl Med. 2015. PMID: 25888743 Free PMC article.
-
Discovery of SARS-CoV-2 papain-like protease (PLpro) inhibitors with efficacy in a murine infection model.Sci Adv. 2024 Aug 30;10(35):eado4288. doi: 10.1126/sciadv.ado4288. Epub 2024 Aug 30. Sci Adv. 2024. PMID: 39213347 Free PMC article.
References
-
- Bavisotto L, et al. 2007. Antiviral, pharmacokinetic and safety data for GS-9190, a non-nucleoside HCV NS5B polymerase inhibitor, in a phase-1 trial in HCV genotype 1 infected patients. 58th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), 2 to 6 November 2007, Boston, MA http://www.natap.org/2007/AASLD/AASLD_39.htm Accessed 14 March 2011
-
- Bondy SS, Oare DA, Tse WC. June 2011. Pyridazine compound and use thereof. US patent 7,956,184
-
- Brainard DM, et al. 2009. Safety and antiviral activity of NS5B polymerase inhibitor MK-3281 in genotype 1 and 3 HCV-infected patients. 60th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), 30 October to 3 November 2009, Boston, MA http://www.natap.org/2009/AASLD/AASLD_23.htm Accessed 14 March 2011
-
- Brass CA, et al. 2011. Sustained virologic response and boceprevir resistance-associated variants observed in patients infected with HCV genotype 1a/1b when treated with boceprevir plus peginterferon alpha-2b/ribavirin: SVR rates among patients with G1b virus were consistently higher than G1a patients in both SPRINT-2 and RESPOND-2. The International Liver Congress 2011, 46th Annual Meeting of the European Association for the Study of the Liver (EASL), 30 March to 3 April 2011, Berlin, Germany http://www.natap.org/2011/EASL/EASL_96.htm Accessed 20 October 2011
-
- Chauret N, et al. 2009. Preclinical pharmacokinetic and ADME characterization of VCH-222, a novel non-nucleoside HCV NS5B polymerase inhibitor from Vertex/ViroChem. The International Liver Congress 2009, 44th Annual Meeting of the European Association for the Study of the Liver (EASL), 22 to 26 April 2009, Copenhagen, Denmark http://www.natap.org/2009/EASL/EASL_65.htm Accessed 8 June 2011
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

