Pharmacokinetic comparison of two piperaquine-containing artemisinin combination therapies in Papua New Guinean children with uncomplicated malaria
- PMID: 22470119
- PMCID: PMC3370744
- DOI: 10.1128/AAC.06232-11
Pharmacokinetic comparison of two piperaquine-containing artemisinin combination therapies in Papua New Guinean children with uncomplicated malaria
Abstract
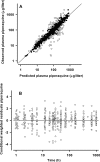
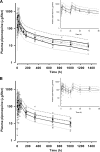
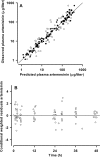
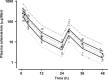
Pharmacokinetic differences between piperaquine (PQ) base and PQ tetraphosphate were investigated in 34 Papua New Guinean children aged 5 to 10 years treated for uncomplicated malaria with artemisinin-PQ (ART-PQ) base or dihydroartemisinin-PQ (DHA-PQ) tetraphosphate. Twelve children received ART-PQ base (two daily doses of 3 mg of ART and 18 mg of PQ base as granules/kg of body weight) as recommended by the manufacturer, with regular clinical assessment and blood sampling over 56 days. PQ concentrations in plasma samples collected from 22 children of similar ages with malaria in a previously published pharmacokinetic study of DHA-PQ tetraphosphate (three daily doses of 2.5 mg of ART and 20 mg of PQ tetraphosphate as tablets/kg of body weight) were available for comparison. The disposition of ART was also assessed in the 12 children who received ART-PQ base. Plasma PQ was assayed by high-performance liquid chromatography with UV detection, and ART was assayed using liquid chromatography-mass spectrometry. Multicompartment pharmacokinetic models for PQ and ART were developed using a population-based approach. ART-PQ base was well tolerated, and initial fever abatement and parasite clearance were prompt. There were no differences between the two treatments in the values for the PQ area under the concentration-time curve from time zero to infinity (AUC(0-∞)), with medians of 49,451 (n = 12) and 44,556 (n = 22) μg · h/liter for ART-PQ base and DHA-PQ tetraphosphate, respectively. Recurrent parasitemia was associated with lower PQ exposure. Using a two-compartment ART model, the median AUC(0-∞) was 1,652 μg · h/liter. There was evidence of autoinduction of ART metabolism (relative bioavailability for the second dose, 0.27). These and previously published data suggest that a 3-day ART-PQ base regimen should be further evaluated, in line with World Health Organization recommendations for all artemisinin combination therapies.
Figures
References
-
- Ahmed T, et al. 2008. Safety, tolerability, and single- and multiple-dose pharmacokinetics of piperaquine phosphate in healthy subjects. J. Clin. Pharmacol. 48:166–175 - PubMed
-
- Anderson BJ, Holford NH. 2009. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab. Pharmacokinet. 24:25–36 - PubMed
-
- Ashley EA, et al. 2007. How much fat is necessary to optimize lumefantrine oral bioavailability? Trop. Med. Int. Health 12:195–200 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

