Cellular Links between Neuronal Activity and Energy Homeostasis
- PMID: 22470340
- PMCID: PMC3308331
- DOI: 10.3389/fphar.2012.00043
Cellular Links between Neuronal Activity and Energy Homeostasis
Abstract
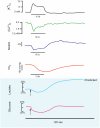
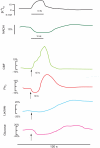
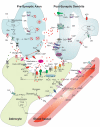
Neuronal activity, astrocytic responses to this activity, and energy homeostasis are linked together during baseline, conscious conditions, and short-term rapid activation (as occurs with sensory or motor function). Nervous system energy homeostasis also varies during long-term physiological conditions (i.e., development and aging) and with adaptation to pathological conditions, such as ischemia or low glucose. Neuronal activation requires increased metabolism (i.e., ATP generation) which leads initially to substrate depletion, induction of a variety of signals for enhanced astrocytic function, and increased local blood flow and substrate delivery. Energy generation (particularly in mitochondria) and use during ATP hydrolysis also lead to considerable heat generation. The local increases in blood flow noted following neuronal activation can both enhance local substrate delivery but also provides a heat sink to help cool the brain and removal of waste by-products. In this review we highlight the interactions between short-term neuronal activity and energy metabolism with an emphasis on signals and factors regulating astrocyte function and substrate supply.
Keywords: ATP; NADH; cerebral metabolism; hippocampus; lactate; neuronal metabolism; oxygen; pyruvate.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

