Cytoplasmic domains and voltage-dependent potassium channel gating
- PMID: 22470342
- PMCID: PMC3311039
- DOI: 10.3389/fphar.2012.00049
Cytoplasmic domains and voltage-dependent potassium channel gating
Abstract
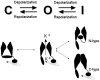
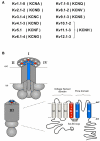
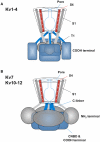
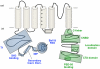
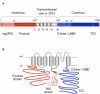
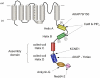
The basic architecture of the voltage-dependent K(+) channels (Kv channels) corresponds to a transmembrane protein core in which the permeation pore, the voltage-sensing components and the gating machinery (cytoplasmic facing gate and sensor-gate coupler) reside. Usually, large protein tails are attached to this core, hanging toward the inside of the cell. These cytoplasmic regions are essential for normal channel function and, due to their accessibility to the cytoplasmic environment, constitute obvious targets for cell-physiological control of channel behavior. Here we review the present knowledge about the molecular organization of these intracellular channel regions and their role in both setting and controlling Kv voltage-dependent gating properties. This includes the influence that they exert on Kv rapid/N-type inactivation and on activation/deactivation gating of Shaker-like and eag-type Kv channels. Some illustrative examples about the relevance of these cytoplasmic domains determining the possibilities for modulation of Kv channel gating by cellular components are also considered.
Keywords: activation/deactivation gating; cytoplasmic domains; inactivation gating; potassium channel; structure–function relationships; voltage-dependent gating.
Figures
References
LinkOut - more resources
Full Text Sources

