Impact of climate trends on tick-borne pathogen transmission
- PMID: 22470348
- PMCID: PMC3313475
- DOI: 10.3389/fphys.2012.00064
Impact of climate trends on tick-borne pathogen transmission
Abstract
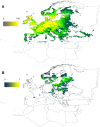
Recent advances in climate research together with a better understanding of tick-pathogen interactions, the distribution of ticks and the diagnosis of tick-borne pathogens raise questions about the impact of environmental factors on tick abundance and spread and the prevalence and transmission of tick-borne pathogens. While undoubtedly climate plays a role in the changes in distribution and seasonal abundance of ticks, it is always difficult to disentangle factors impacting on the abundance of tick hosts from those exerted by human habits. All together, climate, host abundance, and social factors may explain the upsurge of epidemics transmitted by ticks to humans. Herein we focused on tick-borne pathogens that affect humans with epidemic potential. Borrelia burgdorferi s.l. (Lyme disease), Anaplasma phagocytophilum (human granulocytic anaplasmosis), and tick-borne encephalitis virus (tick-borne encephalitis) are transmitted by Ixodes spp. Crimean-Congo hemorrhagic fever virus (Crimean-Congo hemorrhagic fever) is transmitted by Hyalomma spp. In this review, we discussed how vector tick species occupy the habitat as a function of different climatic factors, and how these factors impact on tick survival and seasonality. How molecular events at the tick-pathogen interface impact on pathogen transmission is also discussed. Results from statistically and biologically derived models are compared to show that while statistical models are able to outline basic information about tick distributions, biologically derived models are necessary to evaluate pathogen transmission rates and understand the effect of climatic variables and host abundance patterns on pathogen transmission. The results of these studies could be used to build early alert systems able to identify the main factors driving the subtle changes in tick distribution and seasonality and the prevalence of tick-borne pathogens.
Keywords: Anaplasma; Borrelia; climate; genetics; model; tick; virus.
Figures

References
-
- Alekseev A. N., Chunikhin S. P., Rukhkyan M. Y., Stefutkina L. F. (1991). Possible role of Ixodidae salivary gland substrate as an adjuvant enhancing arbovirus transmission. Med. Parazitol. (Mosk) 1, 28–31 - PubMed
-
- Apanaskevich D. A. (2004). Host-parasite relationships of the genus Hyalomma Koch, 1844 (Acari, Ixodidae) and their connection with microevolutionary process. Parazitologia 38, 515–523 - PubMed
-
- Apanaskevich D. A., Horak I. V. (2008). The genus Hyalomma Koch, 1844: V- re-evaluation of the taxonomic rank of taxa comprising the H. (Euhyalomma) marginatum Koch complex of species (Acari: Ixodidae) with redescriptions of all parasitic stages and notes on biology. Int. J. Acarol. 34, 13–4210.1080/01647950808683704 - DOI
-
- Bacon R. M., Kugler K. J., Mead P. S. (2008). Surveillance for Lyme disease United States, 1992–2006. MMWR Surveill. Summ. 57, 1–9 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

