Early developing pig embryos mediate their own environment in the maternal tract
- PMID: 22470458
- PMCID: PMC3314662
- DOI: 10.1371/journal.pone.0033625
Early developing pig embryos mediate their own environment in the maternal tract
Abstract
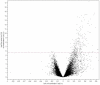
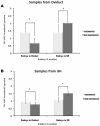
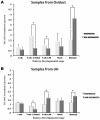
The maternal tract plays a critical role in the success of early embryonic development providing an optimal environment for establishment and maintenance of pregnancy. Preparation of this environment requires an intimate dialogue between the embryo and her mother. However, many intriguing aspects remain unknown in this unique communication system. To advance our understanding of the process by which a blastocyst is accepted by the endometrium and better address the clinical challenges of infertility and pregnancy failure, it is imperative to decipher this complex molecular dialogue. The objective of the present work is to define the local response of the maternal tract towards the embryo during the earliest stages of pregnancy. We used a novel in vivo experimental model that eliminated genetic variability and individual differences, followed by Affymetrix microarray to identify the signals involved in this embryo-maternal dialogue. Using laparoscopic insemination one oviduct of a sow was inseminated with spermatozoa and the contralateral oviduct was injected with diluent. This model allowed us to obtain samples from the oviduct and the tip of the uterine horn containing either embryos or oocytes from the same sow. Microarray analysis showed that most of the transcripts differentially expressed were down-regulated in the uterine horn in response to blastocysts when compared to oocytes. Many of the transcripts altered in response to the embryo in the uterine horn were related to the immune system. We used an in silico mathematical model to demonstrate the role of the embryo as a modulator of the immune system. This model revealed that relatively modest changes induced by the presence of the embryo could modulate the maternal immune response. These findings suggested that the presence of the embryo might regulate the immune system in the maternal tract to allow the refractory uterus to tolerate the embryo and support its development.
Conflict of interest statement
Figures



References
-
- Fazeli A. Maternal communication with gametes and embryos. Theriogenology. 2008;70:1182–1187. - PubMed
-
- Pope W. Embryonic mortality in swine; In: Geisert Rd ZM, editor. Boca Raton: CRS Press; 1994. pp. 53–77.
-
- Simon C, Moreno C, Remohi J, Pellicer A. Molecular interactions between embryo and uterus in the adhesion phase of human implantation. Hum Reprod. 1998;13(Suppl 3):219–232; discussion 233–216. - PubMed
-
- Wolf E, Arnold GJ, Bauersachs S, Beier HM, Blum H, et al. Embryo-maternal communication in bovine - strategies for deciphering a complex cross-talk. Reprod Domest Anim. 2003;38:276–289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

