Induction of apoptosis coupled to endoplasmic reticulum stress in human prostate cancer cells by n-butylidenephthalide
- PMID: 22470469
- PMCID: PMC3314677
- DOI: 10.1371/journal.pone.0033742
Induction of apoptosis coupled to endoplasmic reticulum stress in human prostate cancer cells by n-butylidenephthalide
Abstract
Background: N-butylidenephthalide (BP) exhibits antitumor effect in a variety of cancer cell lines. The objective of this study was to obtain additional insights into the mechanisms involved in BP induced cell death in human prostate cancer cells.
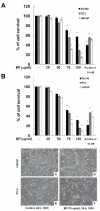
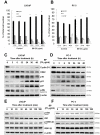
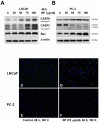
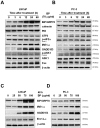
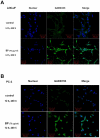
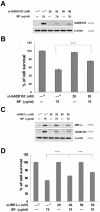
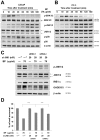
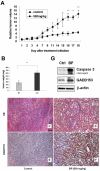
Methods/principal findings: Two human prostate cancer cell lines, PC-3 and LNCaP, were treated with BP, and subsequently evaluated for their viability and cell cycle profiles. BP caused cell cycle arrest and cell death in both cell lines. The G0/G1 phase arrest was correlated with increase levels of CDK inhibitors (p16, p21 and p27) and decrease of the checkpoint proteins. To determine the mechanisms of BP-induced growth arrest and cell death in prostate cancer cell lines, we performed a microarray study to identify alterations in gene expression induced by BP in the LNCaP cells. Several BP-induced genes, including the GADD153/CHOP, an endoplasmic reticulum stress (ER stress)-regulated gene, were identified. BP-induced ER stress was evidenced by increased expression of the downstream molecules GRP78/BiP, IRE1-α and GADD153/CHOP in both cell lines. Blockage of IRE1-α or GADD153/CHOP expression by siRNA significantly reduced BP-induced cell death in LNCaP cells. Furthermore, blockage of JNK1/2 signaling by JNK siRNA resulted in decreased expression of IRE1-α and GADD153/CHOP genes, implicating that BP-induced ER stress may be elicited via JNK1/2 signaling in prostate cancer cells. BP also suppressed LNCaP xenograft tumor growth in NOD-SCID mice. It caused 68% reduction in tumor volume after 18 days of treatment.
Conclusions: Our results suggest that BP can cause G0/G1 phase arrest in prostate cancer cells and its cytotoxicity is mediated by ER stress induction. Thus, BP may serve as an anticancer agent by inducing ER stress in prostate cancer.
Conflict of interest statement
Figures
Similar articles
-
Tanshinone IIA inhibits human prostate cancer cells growth by induction of endoplasmic reticulum stress in vitro and in vivo.Prostate Cancer Prostatic Dis. 2013 Dec;16(4):315-22. doi: 10.1038/pcan.2013.38. Epub 2013 Sep 17. Prostate Cancer Prostatic Dis. 2013. PMID: 24042854
-
Proteomic-based identification of multiple pathways underlying n-butylidenephthalide-induced apoptosis in LNCaP human prostate cancer cells.Food Chem Toxicol. 2013 Sep;59:281-8. doi: 10.1016/j.fct.2013.05.045. Epub 2013 Jun 12. Food Chem Toxicol. 2013. PMID: 23770345
-
Tongguanteng injection exerts anti-osteosarcoma effects through the ER stress-associated IRE1/CHOP pathway.BMC Complement Med Ther. 2024 Nov 16;24(1):400. doi: 10.1186/s12906-024-04689-7. BMC Complement Med Ther. 2024. PMID: 39550552 Free PMC article.
-
Dienogest regulates apoptosis, proliferation, and invasiveness of endometriotic cyst stromal cells via endoplasmic reticulum stress induction.Mol Hum Reprod. 2020 Jan 1;26(1):30-39. doi: 10.1093/molehr/gaz064. Mol Hum Reprod. 2020. PMID: 31814016
-
IRE1 is a promising therapeutic target in pancreatic cancer.Am J Physiol Cell Physiol. 2025 Mar 1;328(3):C806-C824. doi: 10.1152/ajpcell.00551.2024. Epub 2025 Jan 16. Am J Physiol Cell Physiol. 2025. PMID: 39819023 Review.
Cited by
-
Identification and structural-functional analysis of cyclin-dependent kinases of the cattle tick Rhipicephalus (Boophilus) microplus.PLoS One. 2013 Oct 11;8(10):e76128. doi: 10.1371/journal.pone.0076128. eCollection 2013. PLoS One. 2013. PMID: 24146826 Free PMC article.
-
SILAC-Based Mass Spectrometry Analysis Reveals That Epibrassinolide Induces Apoptosis via Activating Endoplasmic Reticulum Stress in Prostate Cancer Cells.PLoS One. 2015 Sep 9;10(9):e0135788. doi: 10.1371/journal.pone.0135788. eCollection 2015. PLoS One. 2015. PMID: 26353013 Free PMC article.
-
Inhibition of breast cancer with transdermal tamoxifen-encapsulated lipoplex.J Nanobiotechnology. 2016 Feb 19;14:11. doi: 10.1186/s12951-016-0163-3. J Nanobiotechnology. 2016. PMID: 26892504 Free PMC article.
-
The Methanol Extract of Angelica sinensis Induces Cell Apoptosis and Suppresses Tumor Growth in Human Malignant Brain Tumors.Evid Based Complement Alternat Med. 2013;2013:394636. doi: 10.1155/2013/394636. Epub 2013 Nov 10. Evid Based Complement Alternat Med. 2013. PMID: 24319475 Free PMC article.
-
Curcumin inhibits proliferation of gastric cancer cells by impairing ATP-sensitive potassium channel opening.World J Surg Oncol. 2014 Dec 19;12:389. doi: 10.1186/1477-7819-12-389. World J Surg Oncol. 2014. PMID: 25523120 Free PMC article.
References
-
- Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12:20–37. - PubMed
-
- Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–667. - PubMed
-
- Wang H, Chen R, Xu H. [Chemical constituents of radix Angelicae Sinensis]. Zhongguo Zhong Yao Za Zhi. 1998;23:167–168. inside backcover. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

