FRET imaging of diatoms expressing a biosilica-localized ribose sensor
- PMID: 22470473
- PMCID: PMC3309933
- DOI: 10.1371/journal.pone.0033771
FRET imaging of diatoms expressing a biosilica-localized ribose sensor
Abstract
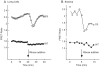
Future materials are envisioned to include bio-assembled, hybrid, three-dimensional nanosystems that incorporate functional proteins. Diatoms are amenable to genetic modification for localization of recombinant proteins in the biosilica cell wall. However, the full range of protein functionalities that can be accommodated by the modified porous biosilica has yet to be described. Our objective was to functionalize diatom biosilica with a reagent-less sensor dependent on ligand-binding and conformational change to drive FRET-based signaling capabilities. A fusion protein designed to confer such properties included a bacterial periplasmic ribose binding protein (R) flanked by CyPet (C) and YPet (Y), cyan and yellow fluorescent proteins that act as a FRET pair. The structure and function of the CRY recombinant chimeric protein was confirmed by expression in E. coli prior to transformation of the diatom Thalassiosira pseudonana. Mass spectrometry of the recombinant CRY showed 97% identity with the deduced amino acid sequence. CRY with and without an N-terminal Sil3 tag for biosilica localization exhibited characteristic ribose-dependent changes in FRET, with similar dissociation constants of 123.3 µM and 142.8 µM, respectively. The addition of the Sil3 tag did not alter the affinity of CRY for the ribose substrate. Subsequent transformation of T. pseudonana with a vector encoding Sil3-CRY resulted in fluorescence localization in the biosilica and changes in FRET in both living cells and isolated frustules in response to ribose. This work demonstrated that the nano-architecture of the genetically modified biosilica cell wall was able to support the functionality of the relatively complex Sil3-CyPet-RBP-YPet fusion protein with its requirement for ligand-binding and conformational change for FRET-signal generation.
Conflict of interest statement
Figures





References
-
- Chen J, Doumamidis H, Lyons K, Murday J, Roco MC. Manufacturing at the Nanoscale. National Nanotechnology Initiative Workshop Report 2007
-
- Gordon R, Parkinson J. Potential roles for diatomists in nanotechnology. Journal of Nanoscience and Nanotechnology. 2005;5:35–40. - PubMed
-
- Chang CH, Wang W, Gutu T, Gale DK, Jiao J, et al. Self-Assembly of Nanostructured Diatom Microshells into Patterned Arrays Assisted by Polyelectrolyte Multilayer Deposition and Inkjet Printing. Journal of the American Chemical Society. 2009;131:4178. - PubMed
-
- Kent MS, Murton JK, Satija S, Kuzmenko I, Simmons BA. Nanosilica Formation at Lipid Membranes Induced by Silaffin Peptides. Structure-Property Relationships in Biomineralized and Biomimetic Composites. 2009;1187:95–100.
-
- Kröger N, Deutzmann R, Sumper M. Polycationic peptides from diatom biosilica that direct silica nanosphere formation. Science. 1999;286:1129–1132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

