Functionality of CD4+ and CD8+ T cells from tonsillar tissue
- PMID: 22471281
- PMCID: PMC3390521
- DOI: 10.1111/j.1365-2249.2012.04573.x
Functionality of CD4+ and CD8+ T cells from tonsillar tissue
Abstract
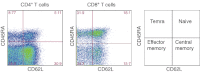
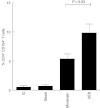
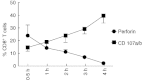
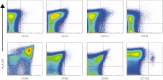
For many years, tonsillectomy has been used routinely in children to treat chronic or recurrent acute tonsillitis. Palatine tonsils are secondary lymphoid organs and the major barrier protecting the digestive and respiratory tracts from potential invasive microorganisms. They have been used as sources of lymphoid tissue; however, despite the hundreds of papers published on tonsillectomy, no studies addressing the functionality of the CD4(+) and CD8(+) T cells from chronically infected tonsils have yet been published. The aim of this study was to analyse the functionality of the CD4(+) and CD8(+) T cells with respect to tonsillar tissue. We used an affordable approach to measure the frequency of antigen-specific CD4(+) T cells, the direct ex-vivo cytotoxicity of CD8(+) T cells, memory T cell phenotype, cytokine profile and DC phenotype. Our results demonstrate that CD4(+) and CD8(+) T cells from tonsillar tissue are totally functional, as shown by their ability to produce cytokines, to degranulate and to differentiate into effector-memory T cells.
© 2012 The Authors;Clinical and Experimental Immunology © 2012 British Society for Immunology.
Figures

Similar articles
-
Functional characterization of T-cells from palatine tonsils in patients with chronic tonsillitis.PLoS One. 2017 Sep 6;12(9):e0183214. doi: 10.1371/journal.pone.0183214. eCollection 2017. PLoS One. 2017. PMID: 28877231 Free PMC article.
-
Unique phenotype of human tonsillar and in vitro-induced FOXP3+CD8+ T cells.J Immunol. 2009 Feb 15;182(4):2124-30. doi: 10.4049/jimmunol.0802271. J Immunol. 2009. PMID: 19201865
-
Isolation of mononuclear cells from tonsillar tissue.Curr Protoc Immunol. 2009 Aug;Chapter 7:7.8.1-7.8.4. doi: 10.1002/0471142735.im0708s86. Curr Protoc Immunol. 2009. PMID: 19653208
-
Modified Vaccinia Ankara-Vectored Vaccine Expressing Nucleoprotein and Matrix Protein 1 (M1) Activates Mucosal M1-Specific T-Cell Immunity and Tissue-Resident Memory T Cells in Human Nasopharynx-Associated Lymphoid Tissue.J Infect Dis. 2020 Aug 4;222(5):807-819. doi: 10.1093/infdis/jiz593. J Infect Dis. 2020. PMID: 31740938 Free PMC article.
-
Lymphocyte subsets in human tonsils: the effect of age and infection.Pediatr Allergy Immunol. 1998 Aug;9(3):161-7. doi: 10.1111/j.1399-3038.1998.tb00364.x. Pediatr Allergy Immunol. 1998. PMID: 9814732
Cited by
-
Live Attenuated Influenza Vaccine in Children Induces B-Cell Responses in Tonsils.J Infect Dis. 2016 Sep 1;214(5):722-31. doi: 10.1093/infdis/jiw230. Epub 2016 May 30. J Infect Dis. 2016. PMID: 27247344 Free PMC article.
-
In vitro cell culture model of human nasal-associated lymphoid tissue (NALT) to evaluate the humoral immune response to SARS-CoV-2 spike proteins.Saudi J Biol Sci. 2021 Aug;28(8):4516-4521. doi: 10.1016/j.sjbs.2021.04.051. Epub 2021 Apr 24. Saudi J Biol Sci. 2021. PMID: 33942008 Free PMC article.
-
Functional characterization of T-cells from palatine tonsils in patients with chronic tonsillitis.PLoS One. 2017 Sep 6;12(9):e0183214. doi: 10.1371/journal.pone.0183214. eCollection 2017. PLoS One. 2017. PMID: 28877231 Free PMC article.
-
Immune responses after live attenuated influenza vaccination.Hum Vaccin Immunother. 2018 Mar 4;14(3):571-578. doi: 10.1080/21645515.2017.1377376. Epub 2018 Jan 3. Hum Vaccin Immunother. 2018. PMID: 28933664 Free PMC article. Review.
-
Innate adaptive immune cell dynamics in tonsillar tissues during chronic SIV infection.Front Immunol. 2023 Aug 21;14:1201677. doi: 10.3389/fimmu.2023.1201677. eCollection 2023. Front Immunol. 2023. PMID: 37671159 Free PMC article.
References
-
- Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol. 2003;3:361–70. - PubMed
-
- Dorner T, Radbruch A. Antibodies and B cell memory in viral immunity. Immunity. 2007;27:384–92. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. - PubMed
-
- Itano AA, McSorley SJ, Reinhardt RL, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

