The Tat protein of human immunodeficiency virus-1 enhances hepatitis C virus replication through interferon gamma-inducible protein-10
- PMID: 22471703
- PMCID: PMC3350415
- DOI: 10.1186/1471-2172-13-15
The Tat protein of human immunodeficiency virus-1 enhances hepatitis C virus replication through interferon gamma-inducible protein-10
Abstract
Background: Co-infection with human immunodeficiency virus-1 (HIV-1) and hepatitis C virus (HCV) is associated with faster progression of liver disease and an increase in HCV persistence. However, the mechanism by which HIV-1 accelerates the progression of HCV liver disease remains unknown.
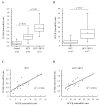
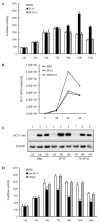
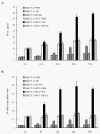
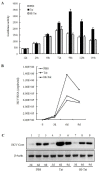
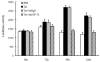
Results: HIV-1/HCV co-infection is associated with increased expression of interferon gamma-induced protein-10 (IP-10) mRNA in peripheral blood mononuclear cells (PBMCs). HCV RNA levels were higher in PBMCs of patients with HIV-1/HCV co-infection than in patients with HCV mono-infection. HIV-1 Tat and IP-10 activated HCV replication in a time-dependent manner, and HIV-1 Tat induced IP-10 production. In addition, the effect of HIV-1 Tat on HCV replication was blocked by anti-IP-10 monoclonal antibody, demonstrating that the effect of HIV-1 Tat on HCV replication depends on IP-10. Taken together, these results suggest that HIV-1 Tat protein activates HCV replication by upregulating IP-10 production.
Conclusions: HIV-1/HCV co-infection is associated with increased expression of IP-10 mRNA and replication of HCV RNA. Furthermore, both HIV-1 Tat and IP-10 activate HCV replication. HIV-1 Tat activates HCV replication by upregulating IP-10 production. These results expand our understanding of HIV-1 in HCV replication and the mechanism involved in the regulation of HCV replication mediated by HIV-1 during co-infection.
Figures
Similar articles
-
HIV Vpr protein upregulates microRNA-122 expression and stimulates hepatitis C virus replication.J Gen Virol. 2015 Aug;96(8):2453-2463. doi: 10.1099/vir.0.000169. Epub 2015 Apr 28. J Gen Virol. 2015. PMID: 25920531 Free PMC article.
-
Crosstalk between HIV and hepatitis C virus during co-infection.BMC Med. 2012 Apr 3;10:32. doi: 10.1186/1741-7015-10-32. BMC Med. 2012. PMID: 22472061 Free PMC article.
-
Effects of HCV on basal and tat-induced HIV LTR activation.PLoS One. 2013 Jun 10;8(6):e64956. doi: 10.1371/journal.pone.0064956. Print 2013. PLoS One. 2013. PMID: 23762271 Free PMC article.
-
MicroRNAs, hepatitis C virus, and HCV/HIV-1 co-infection: new insights in pathogenesis and therapy.Viruses. 2012 Oct 26;4(11):2485-513. doi: 10.3390/v4112485. Viruses. 2012. PMID: 23202492 Free PMC article. Review.
-
MicroRNAs, HIV and HCV: a complex relation towards pathology.Rev Med Virol. 2016 May;26(3):197-215. doi: 10.1002/rmv.1881. Epub 2016 Apr 5. Rev Med Virol. 2016. PMID: 27059433 Review.
Cited by
-
Predictors of Chronic Hepatitis C Evolution in HIV Co-Infected Patients From Romania.Hepat Mon. 2013 Feb 28;13(2):e8611. doi: 10.5812/hepatmon.8611. Print 2013 Feb. Hepat Mon. 2013. PMID: 23613686 Free PMC article.
-
HIV-1 coinfection profoundly alters intrahepatic chemokine but not inflammatory cytokine profiles in HCV-infected subjects.PLoS One. 2014 Feb 6;9(2):e86964. doi: 10.1371/journal.pone.0086964. eCollection 2014. PLoS One. 2014. PMID: 24516541 Free PMC article.
-
High IP-10 levels decrease T cell function in HIV-1-infected individuals on ART.J Leukoc Biol. 2014 Dec;96(6):1055-63. doi: 10.1189/jlb.3A0414-232RR. Epub 2014 Aug 25. J Leukoc Biol. 2014. PMID: 25157027 Free PMC article.
-
HIV Vpr protein upregulates microRNA-122 expression and stimulates hepatitis C virus replication.J Gen Virol. 2015 Aug;96(8):2453-2463. doi: 10.1099/vir.0.000169. Epub 2015 Apr 28. J Gen Virol. 2015. PMID: 25920531 Free PMC article.
-
Direct effects of hepatitis C virus on the lymphoid cells.World J Gastroenterol. 2013 Nov 28;19(44):7889-95. doi: 10.3748/wjg.v19.i44.7889. World J Gastroenterol. 2013. PMID: 24307783 Free PMC article. Review.
References
-
- Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, Vidaud M, Bricaire F, Opolon P, Katlama C. et al.Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30:1054–1058. doi: 10.1002/hep.510300409. - DOI - PubMed
-
- Macias J, Berenguer J, Japon MA, Giron JA, Rivero A, Lopez-Cortes LF, Moreno A, Gonzalez-Serrano M, Iribarren JA, Ortega E. et al.Fast fibrosis progression between repeated liver biopsies in patients coinfected with human immunodeficiency virus/hepatitis C virus. Hepatology. 2009;50:1056–1063. doi: 10.1002/hep.23136. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

