Mutations in the potassium channel subunit KCNE1 are associated with early-onset familial atrial fibrillation
- PMID: 22471742
- PMCID: PMC3359244
- DOI: 10.1186/1471-2350-13-24
Mutations in the potassium channel subunit KCNE1 are associated with early-onset familial atrial fibrillation
Abstract
Background: Atrial fibrillation (AF) is the most common arrhythmia. The potassium current IKs is essential for cardiac repolarization. Gain-of-function mutations in KV7.1, the pore-forming α-subunit of the IKs channel, have been associated with AF. We hypothesized that early-onset lone AF is associated with mutations in the IKs channel regulatory subunit KCNE1.
Methods: In 209 unrelated early-onset lone AF patients (< 40 years) the entire coding sequence of KCNE1 was bidirectionally sequenced. We analyzed the identified KCNE1 mutants electrophysiologically in heterologous expression systems.
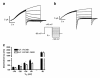
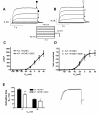
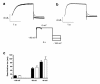
Results: Two non-synonymous mutations G25V and G60D were found in KCNE1 that were not present in the control group (n = 432 alleles) and that have not previously been reported in any publicly available databases or in the exom variant server holding exom data from more than 10.000 alleles. Proband 1 (female, age 45, G25V) had onset of paroxysmal AF at the age of 39 years. Proband 2 (G60D) was diagnosed with lone AF at the age of 33 years. The patient has inherited the mutation from his mother, who also has AF. Both probands had no mutations in genes previously associated with AF. In heterologous expression systems, both mutants showed significant gain-of-function for IKs both with respect to steady-state current levels, kinetic parameters, and heart rate-dependent modulation.
Conclusions: Mutations in KV7.1 leading to gain-of-function of IKs current have previously been described in lone AF, yet this is the first time a mutation in the beta-subunit KCNE1 is associated with the disease. This finding further supports the hypothesis that increased potassium current enhances AF susceptibility.
Figures


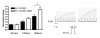
References
-
- Benjamin EJ, Wolf PA, D'Agostino RB. et al. Impact of atrial fibrillation on the risk of death: the Framingham heart study. Circulation. 1998;98(10):946–952. - PubMed
-
- Fuster V, Rydén LE, Cannom DS. et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation-excutive summary. Rev Port Cardiol. 2007;26(4):383–446. - PubMed
-
- Go AS, Hylek EM, Phillips KA. et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. - DOI - PubMed
-
- Psaty BM, Manolio TA, Kuller LH. et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96(7):2455–2461. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

