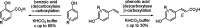Regioselective enzymatic carboxylation of phenols and hydroxystyrene derivatives
- PMID: 22471935
- PMCID: PMC3593611
- DOI: 10.1021/ol300385k
Regioselective enzymatic carboxylation of phenols and hydroxystyrene derivatives
Abstract
The enzymatic carboxylation of phenol and styrene derivatives using (de)carboxylases in carbonate buffer proceeded in a highly regioselective fashion: Benzoic acid (de)carboxylases selectively formed o-hydroxybenzoic acid derivatives, phenolic acid (de)carboxylases selectively acted at the β-carbon atom of styrenes forming (E)-cinnamic acids.
© 2012 American Chemical Society
Figures
Similar articles
-
Pushing the equilibrium of regio-complementary carboxylation of phenols and hydroxystyrene derivatives.J Biotechnol. 2013 Nov;168(3):264-70. doi: 10.1016/j.jbiotec.2013.07.017. Epub 2013 Jul 20. J Biotechnol. 2013. PMID: 23880442
-
Amine-Mediated Enzymatic Carboxylation of Phenols Using CO2 as Substrate Increases Equilibrium Conversions and Reaction Rates.Biotechnol J. 2017 Dec;12(12). doi: 10.1002/biot.201700332. Epub 2017 Sep 25. Biotechnol J. 2017. PMID: 28862371
-
Sensitivity to vinyl phenol derivatives produced by phenolic acid decarboxylase activity in Escherichia coli and several food-borne Gram-negative species.Appl Microbiol Biotechnol. 2013 Sep;97(17):7853-64. doi: 10.1007/s00253-013-5072-x. Epub 2013 Jul 12. Appl Microbiol Biotechnol. 2013. PMID: 23846865
-
Plant-derived and dietary phenolic cinnamic acid derivatives: Anti-inflammatory properties.Food Chem. 2024 Nov 30;459:140080. doi: 10.1016/j.foodchem.2024.140080. Epub 2024 Jun 26. Food Chem. 2024. PMID: 38986205 Review.
-
Non-Oxidative Enzymatic (De)Carboxylation of (Hetero)Aromatics and Acrylic Acid Derivatives.Adv Synth Catal. 2019 Jun 6;361(11):2402-2420. doi: 10.1002/adsc.201900275. Epub 2019 May 17. Adv Synth Catal. 2019. PMID: 31379472 Free PMC article. Review.
Cited by
-
De novo biosynthesis of 2-hydroxyterephthalic acid, the monomer for high-performance hydroxyl modified PBO fiber, by enzymatic Kolbe-Schmitt reaction with CO2 fixation.Biotechnol Biofuels Bioprod. 2023 Nov 20;16(1):179. doi: 10.1186/s13068-023-02413-0. Biotechnol Biofuels Bioprod. 2023. PMID: 37986026 Free PMC article.
-
Synthetic Enzyme-Catalyzed CO2 Fixation Reactions.ChemSusChem. 2021 Apr 22;14(8):1781-1804. doi: 10.1002/cssc.202100159. Epub 2021 Mar 10. ChemSusChem. 2021. PMID: 33631048 Free PMC article. Review.
-
Biocatalysis for the application of CO2 as a chemical feedstock.Beilstein J Org Chem. 2015 Dec 1;11:2370-87. doi: 10.3762/bjoc.11.259. eCollection 2015. Beilstein J Org Chem. 2015. PMID: 26734087 Free PMC article. Review.
-
Understanding a substrate's product regioselectivity in a family of enzymes: a case study of acetaminophen binding in cytochrome P450s.PLoS One. 2014 Feb 3;9(2):e87058. doi: 10.1371/journal.pone.0087058. eCollection 2014. PLoS One. 2014. PMID: 24498291 Free PMC article.
-
Terminal Alkenes from Acrylic Acid Derivatives via Non-Oxidative Enzymatic Decarboxylation by Ferulic Acid Decarboxylases.ChemCatChem. 2018 Sep 7;10(17):3736-3745. doi: 10.1002/cctc.201800643. Epub 2018 Jul 17. ChemCatChem. 2018. PMID: 30333895 Free PMC article.
References
-
-
The major intermediates derived from crude oil encompass methanol, alkenes (ethene, propene, butene, butadiene), and aromatics (benzene, toluene, xylenes).
-
-
- Behr A.Angewandte Homogene Katalyse; Wiley-VCH: Weinheim, 2008; Chapter 28, pp 441–464.
- Aresta M.; Dibenedetto A. Dalton Trans. 2007, 2975–2992. - PubMed
- Huang K.; Sun C.-L.; Shi Z.-J. Chem. Soc. Rev. 2011, 40, 2435–2452. - PubMed
- Baiker A. Appl. Organomet. Chem. 2000, 14, 751–762.
- Omae I. Catal. Today 2006, 115, 33–52.
- Zevenhoven R.; Eloneva S.; Teir S. Catal. Today 2006, 115, 73–79.
- Aresta M., Ed. Carbon Dioxide as Chemical Feedstock; Wiley-VCH: Weinheim, 2010.
-
- Zhang L.; Cheng J.; Ohishi T.; Hou Z. Angew. Chem., Int. Ed. 2010, 49, 8670–8673. - PubMed
- Bernhardt S.; Metzger A.; Knochel P. Synthesis 2010, 3802–3810.
-
- Zhang Y.-G.; Riduan S. N. Angew. Chem., Int. Ed. 2011, 50, 6210–6212. - PubMed
-
- Decortes A.; Castilla A. M.; Kleij A. W. Angew. Chem., Int. Ed. 2010, 49, 9822–9837. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources